Abstract
Purpose
Physicians and oncology nurses must continue to update their knowledge on treatment and treatment-related side effects, while searching for effective methods to prevent or manage side effects. The objective of our study was to describe the incidence and response to treatment of the hand-foot syndrome (HFS) and the compliance with treatment of patients with stage IIB, IIIA, IIIB, and IIIC colon cancer that were treated with capecitabine alone as adjuvant therapy.
Materials and Methods
Between September 2005 and September 2006, 84 patients fulfilled the inclusion criteria and were included in this retrospective analysis of prospectively collected data.
Results
The treatment compliance rate was 90.5% (76 out of the 84 patients). The HFS developed in 65 patients (77.4%). Thirty-three patients (50.7%) had grade 1 HFS, 22 patients (33.8%) had grade 2 HFS and 10 patients (15.5%) had grade 3 HFS, as their most severe episode. For Grade 1 patients, the dose was maintained, and skin barrier cream and moist exposed burn ointment (MEBO) were applied. For Grade 2 patients, either the dose was maintained or 25% of the dose was reduced; MEBO and supportive care were provided. For Grade 3 patients, one cycle of chemotherapy was interrupted followed by dose adjustment; MEBO and supportive care were provided.
Oral chemotherapeutic agents play an important role in the management of many different types of cancer; and it is likely that the use of these drugs will continue to grow. The use of 5-fluorouracil (5-FU) has been an integral part of many cytotoxic regimens for the treatment of malignant disease over the past four decades.1 Oral fluropyrimidine is also called capecitabine; and it has been shown to be effective against colon cancer with a disease-free survival that is at least equivalent to that of fluorouracil-plus-leucovorin treatment.2
Physicians and oncology nurses must continuously update their knowledge on current treatments and treatment-related side effects; and they must search for effective methods to prevent and/or manage the side effects that develop. In addition, they are responsible for educating their patients about the available regimens and providing them with accurate information regarding: 1) the drug names, dosages and when, where, and how treatment will be administered, 2) the anticipated side effects and 3) the methods used to prevent side effects as well as the methods used to treat the complications when they do occur.
Although capecitabine is generally well tolerated, many patients experience side effects that can be controlled by additional treatments provided by physicians and/or nurses. The hand-foot syndrome (HFS) is the only clinical adverse event that commonly occurs with capecitabine treatment. HFS is also referred to as palmar-plantar erythrodysesthesia; and it warrants special attention because it is the most common dose-limiting toxicity, and the only clinically significant adverse event that frequently occurs with the administration of capecitabine when compared to 5-FU/ leucovorin intravenous administration.2
HFS is a distinctive and relatively common toxic reaction related to some chemotherapeutic agents.3 HFS was initially described by Zuehlke in 1974 in association with mitone therapy for a hypernephroma.4 In 1980, it was referred to as chemotherapy-induced acral erythema and appeared to be associated with cutaneous exposure to a variety of chemotherapy drugs.4 Most patients present with a prodrome of dysesthesia, usually a tingling sensation of the palms and soles. In a few days, the condition progresses to a burning pain sensation in conjunction with a well-defined swelling and erythema.3 If the offending drug is not adjusted, there may be skin breakdown, and desquamation, bullous formation or secondary infections can occur in severe cases. Therefore, HFS can be a major cause of reduced compliance and quality of life.
The objective of this study was to determine the incidence and response to treatment of HFS, and the compliance with treatment, in patients with stage IIB, IIIA, IIIB, and IIIC colon cancer that were treated with capecitabine alone as adjuvant therapy.
Between September 2005 and September 2006, a total of 420 patients underwent colectomy for colon cancer at Samsung Medical Center. The inclusion criteria for the study were 1) pathologic stage IIA, IIIA, IIIB and IIIC disease according to the TNM classification as proposed by the American Joint Committee of Cancer 6th edition,5 2) patients who underwent capecitabine monotherapy with completion of at least one cycle, 3) an age ≥ 18 years and 4) an Eastern Cooperative Oncology Group (ECOG) performance status of two or less. Eighty-four patients fulfilled the inclusion criteria and they were included in this retrospective analysis of the prospectively collected data.
Capecitabine was initially administered at 2500 mg/m2/day in two daily doses for two weeks, followed by one week of rest. These cycles were repeated every 21 days for eight cycles (24 weeks) until disease recurrence, occurrence of severe toxicity, severe intercurrent illness, or voluntary withdrawal. The patients were clinically evaluated for adverse events (especially HFS), and laboratory data were recorded after the first cycle and at the completion of two cycles thereafter.
Surveillance for response was assessed every three weeks until the end of the eighth cycle. The patients were examined before each cycle of treatment. Physical examination, the serum CEA level (carcino-embryonic antigen) as well as the serum chemistries and blood counts were obtained before each cycle of therapy for the first two cycles and every two cycles thereafter. Chest X-ray and spiral abdominal computed tomography (CT) scanning were performed every six months for the first three years and annually thereafter. Colonoscopy was performed every year for the first five years.
An oncology nurse enrolled subjects during the first round of treatment and collected data on the management of HFS; the data included five interviews and four phone-call audits. Oncology nurses monitored the initial treatment cycle, 1) to educate the patients, pre-medication, 2) to carry out the initial assessment, 3) to explain HFS, 4) and to provide the patients with an information booklet with appropriate phone numbers for contact. From the second to the eighth cycle, 1) the nurse checked for the occurrence of HFS (e.g., time and grade) by interviewing the patients in the outpatient department, 2) managed the HFS, 3) collected data from the interviews in the medical record, 4) and followed up on each cycle by telephone.
The HFS was recorded according to the HFS grading scale (Table 1).6 For Grade 1 patient, dose was maintained and skin barrier cream and moist exposed burn ointment (MEBO) were applied. For Grade 2 patient, either dose was maintained or 25% of dose was reduced. MEBO and Supportive care were applied. For Grade 3 patient, one cycle was interrupted followed by dose adjustment. MEBO and supportive care were applied. Application of the Skin barrier cream was ceased when Grade 2 due to friction accompanied with the cream (Fig. 1).
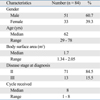
A total of 84 patients, including 51 men and 33 women, were included in the analysis. The median age was 62 (range: 29-78 years). The patient characteristics are presented in Table 2. All patients received capecitabine for at least one cycle. The median number of cycles received was eight cycles.
The treatment compliance rate was 90.5% (76 out of the 84 patients). Eight patients (9.5%) among the 84 discontinued treatment. The reasons for the discontinuation of treatment were "nausea" in 25% (2/8), "patient request" in 25.5% (2/8), "metastasis to another organ" in 25.0% (2/8), "hand-foot syndrome" in 12.5% (1/8), and "neutropenia" in 12.5% (1/8).
Mild to severe adverse events were reported for 84.5% (71 of the 84 patients). The hand-foot syndrome was the most common adverse side effect. The incidence of toxicity is listed in Table 3. The most frequently observed hematological adverse event was leucocytopenia; however, no patient had an episode of neutropenic fever. No patient received prophylactic or therapeutic granulocyte colony stimulating factor during this study. Gastrointestinal adverse events were observed more frequently. Eight patients reported diarrhea (9.5%); and nausea and vomiting were observed in 29 patients (34.5%). With respect to the laboratory values, increased transaminase activity was noted in 10 patients (11.9%) and one patient (1.2%) developed hyperbilirubinemia. The hand-foot syndrome developed in 65 patients (77.4%). The clinical manifestations of the HFS are presented in Fig. 2. There were 90.5% of the patients that completed the full eight cycles.
For analysis of the severity of the HFS, only the most severe episode of HFS was considered for each patient. The grade distribution of the worst episodes among 65 patients with HFS is presented in Table 4. Thirty-three patients (50.7%) had grade 1 HFS, 22 patients (33.8%) had grade 2 HFS and 10 patients (15.5%) had grade 3 HFS as their most severe episode.
The HFS was evaluated at the time of the development of the first episode and at the time of the worst episode. Among the 65 patients who had the HFS, 45 patients (73.9%) had their first episode after the second or more cycles. The most severe episode occurred during or after the fourth cycle in 39 patients (60.0%). The first or worst episode of HFS occurred less frequently within the first two weeks of initiating chemotherapy. The HFS grade was higher in the feet than the hands.
At the time of the first treatment medication, the patients were educated using a booklet for the oral medication used for HFS management by an oncology nurse. The HFS was managed by monitoring the side effects and the medication. In addition, the patients were warned to take special care of their affected areas. They applied skin emollient and skin barrier cream. For the cases with Grade 1 HFS, the treatment continued using the original dose with the application of a skin barrier cream and MEBO. For Grade 2 HFS, the original dose was maintained with application of MEBO and supportive care in 13 patients; for nine patients, 75% of the dosage was maintained with application of MEBO and supportive care, according to the condition of the patient. For grade 3 HFS, the chemotherapy was interrupted for one cycle followed by adjustment of the medication to 75% of the original dosage, and continued with application of MEBO and supportive care in seven patients; for two patients, the original dose was continued with application of MEBO and supportive care, and one patient discontinued treatment at the last cycle (the eighth cycle) (Figs. 3 and 4).
Almost one million patients are diagnosed with colorectal cancer every year, and half a million deaths from this neoplasm occur annually worldwide.7,8 Each year, approximately 230,000 patients with colon cancer are eligible for adjuvant therapy.7,8 Survival advantages have been demonstrated with the administration of bolus intravenous fluorouracil plus leucovorin according to the Mayo Clinic regimen (five days every month for six months) or the Rosewell Park regimen (weekly bolus, six of every eight weeks for eight months); this has been the standard care for adjuvant treatment of colon cancer since the 1990s.9 However, with the advent of the new oral fluropyrimidine, capecitabine has been an effective alternative to intravenous fluorouracil plus leucovorin for the adjuvant treatment of colon cancer with significantly fewer adverse events.2
Capecitabine is taken in the outpatient setting; it is difficult to monitor treatment compliance and to detect adverse events when compared to the IV chemotherapeutic agents. HFS is a major adverse event that can interfere with the general activities of daily living; therefore, intensive management and care by medical personnel are necessary to treat HFS.
The mechanism responsible for HFS is unknown. For patients receiving 5-FU, HFS is dose-dependent and it is probably related to the accumulation of the drug in the skin. One theory proposed to explain capecitabine-associated HFS is that specialized skin cells (keratinocytes) might have upgraded levels of the enzyme thymidine phosphorylase.10 Another theory suggests that capecitabine may be eliminated by the eccrine system (sweat secretion), and HFS is caused by an unknown mechanism related to the increased number of eccrine glands present in the hands and feet.11 In relation to this hypothesis, Grade 3 HFS has been observed in the hands, feet, armpits, and genitals of patients with hyperhidrosis. It is thought that more cases occur during the summer season with regard to eccrine gland involvement; but keratin and defection of the epidermis are more likely to occur during the winter season. In this study, the data were not studied by season due to the small number of patients.
Some studies have reported more toxic effects in the areas of the hands compared to the feet; however, more cases were found with the feet affected than the hands in our study. This might be explained by friction, temperature and the characteristics of ambulation after surgery for colorectal cancer. However, the most widely accepted hypothesis is that the capecitabine induces a direct toxic effect against the epidermal cells due to the dose administered. The histopathological findings are similar to other sources of direct toxicity.12
With regard to the safety of capecitabine monotherapy, the majority of adverse events have been manageable and reversible. Compared to a randomized phase III study,13 the most frequently reported adverse reaction in our study was HFS, and the frequency was higher in the present study (77.4% versus 54%, respectively); however, grade 3 HFS was slightly lower (15.5% versus 17%, respectively). The HFS was easily controlled by interruption of the chemotherapy protocol or dose reduction; and there was no case with life threatening toxicity. A total of eight patients (9.5%) discontinued treatment and one of these patients (0.84%) stopped due to the HFS. In a phase III study,13 2% of the patients dropped out of the study because of the HFS, and this rate was higher than the rate in our study.
The cumulative nature of HFS has been difficult to determine. Some investigators have found an increased incidence of HFS,14 while others have found no evidence of cumulative HFS with capecitabine.6 The results of our study demonstrated that most of the first episodes of HFS occurred during the second or more cycles of treatment. This may be due to the cumulative effect of the drug; however, a delayed onset of the distinct toxic effects cannot be completely ruled out. There is consensus among investigators that interruption of the offending drug is the most important step in the treatment of HFS.6,13,15 Other investigators have proposed measures that include cold compresses and topical wound care.16 There have been some studies on the relief of HFS with the use of pyridoxine (vitamin B-6).17-19 We generally recommend petroleum-based ointment and supportive care for grade 1 HFS, and a dose reduction with ointment for grade 2 HFS.
The widely recommended skin barrier creams are used for Grade 0 (preventive use) and Grade 1 HFS; MEBO is used for Grade 2, and a dose reduction with ointment is used for Grade 3. HFS is treated by patient education, preventive management, ointment, conservative management, dose reduction, and interruption of chemotherapy administration, taking into consideration the patients' condition and situation.
In conclusion, HFS is manageable if both patient and oncology care team are educated about the HFS associated with treatment. The active participation of both patient and oncology care team is a key element in the effective management of HFS. Such efforts combined with the proper dose reduction may lead to completion of the recommended cycles, with a reduced frequency of HFS.
Figures and Tables
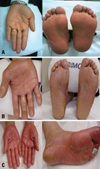 | Fig. 2Clinical manifestations of hand-foot syndrome (HFS). (A) Grade 1 HFS. (B) Characteristic erythema associated with moderate HFS, grade 2. (C) Grade 3 HFS associated with progressed moist desquamation with the patient in an extremely painful and debilitated condition. |
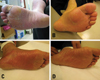 | Fig. 4Management of hand-foot syndrome. (A) Grade 2 HFS; Painful erythema, swelling/ discomfort that affects activity of daily living occurred, and the original dose was maintained with application of MEBO for 1 cycle (3 weeks). (B) After management, Grade 1 HFS; numbness, tingling, painless erythema / discomfort, able to perform activities of daily living. (C) Grade 3 HFS; Pain moist desquamation, ulceration, blistering, and severe pain / unable to perform daily living activities occurred, and the drug administration was interrupted for one cycle (3 weeks). The patient was treated with MEBO. (D) After management, Grade 2 HFS; Painless erythema / discomfort, able to perform daily living activities. |
Table 1
Hand-Foot Syndrome Grading Scale (Palmar-Plantar Erythrodysesthesia)*

*This scale applies only for grading hand-foot syndrome and not for describing any other skin event and/or other cutaneous area. Modified from Blum, et al.6
References
1. Heidelberger C, Chaudhuri NK, Danneberg P, Mooren D, Griesbach L, Duschinsky R, et al. Fluorinated pyrimidines, a new class of tumour-inhibitory compounds. Nature. 1957. 179:663–666.

2. Twelves C, Wong A, Nowacki MP, Abt M, Burris H 3rd, Carrato A, et al. Capecitabine as adjuvant treatment for stage III colon cancer. N Engl J Med. 2005. 352:2696–2704.

3. Nagore E, Insa A, Sanmartín O. Antineoplastic therapy-induced palmar plantar erythrodysesthesia ('hand-foot' syndrome. Incidence, recognition and management. Am J Clin Dermatol. 2000. 1:225–234.

4. Baack BR, Burgdorf WH. Chemotherapy-induced acral erythema. J Am Acad Dermatol. 1991. 24:457–461.

5. Greene FL, Page DL, Fleming ID, Fritz AG, Balch CM, Haller DG, et al. AJCC Cancer Staging Manual. 2002. New York: Springer-Verlag.
6. Blum JL, Jones SE, Buzdar AU, LoRusso PM, Kuter I, Vogel C, et al. Multicenter phase II study of capecitabine in paclitaxel-refractory metastatic breast cancer. J Clin Oncol. 1999. 17:485–493.

7. Ayanian JZ, Zaslavsky AM, Fuchs CS, Guadagnoli E, Creech CM, Cress RD, et al. Use of adjuvant chemotherapy and radiation therapy for colorectal cancer in a population-based cohort. J Clin Oncol. 2003. 21:1293–1300.

8. Benson AB 3rd, Schrag D, Somerfield MR, Cohen AM, Figueredo AT, Flynn PJ, et al. American Society of Clinical Oncology recommendations on adjuvant chemotherapy for stage II colon cancer. J Clin Oncol. 2004. 22:3408–3419.
9. Van Cutsem E, Dicato M, Wils J, Cunningham D, Diaz-Rubio E, Glimelius B, et al. Adjuvant treatment of colorectal cancer (current expert opinion derived from the Third International Conference: Perspectives in Colorectal Cancer, Dublin, 2001). Eur J Cancer. 2002. 38:1429–1436.

10. Asgari MM, Haggerty JG, McNiff JM, Milstone LM, Schwartz PM. Expression and localization of thymidine phosphorylase/platelet-derived endothelial cell growth factor in skin and cutaneous tumors. J Cutan Pathol. 1999. 26:287–294.

11. Mrozek-Orlowski ME, Frye DK, Sanborn HM. Capecitabine: nursing implications of a new oral chemotherapeutic agent. Oncol Nurs Forum. 1999. 26:753–762.
12. Fitzpatrick JE. The cutaneous histopathology of chemotherapeutic reactions. J Cutan Pathol. 1993. 20:1–4.
13. Cassidy J, Twelves C, Van Cutsem E, Hoff P, Bajetta E, Boyer M, et al. First-line oral capecitabine therapy in metastatic colorectal cancer: a favorable safety profile compared with intravenous 5-fluorouracil/leucovorin. Ann Oncol. 2002. 13:566–575.
14. Toxicity of fluorouracil in patients with advanced colorectal cancer: effect of administration schedule and prognostic factors. Meta-Analysis Group In Cancer. J Clin Oncol. 1998. 16:3537–3541.
15. Gerbrecht BM. Current Canadian experience with capecitabine: partnering with patients to optimize therapy. Cancer Nurs. 2003. 26:161–167.
16. Hoff PM, Valero V, Ibrahim N, Willey J, Hortobagyi GN. Hand-foot syndrome following prolonged infusion of high doses of vinorelbine. Cancer. 1998. 82:965–969.

17. Vukelja SJ, Lombardo FA, James WD, Weiss RB. Pyridoxine for the palmar-plantar erythrodysesthesia syndrome. Ann Intern Med. 1989. 111:688–689.





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download