Abstract
Purpose
The risk of hepatocellular carcinoma (HCC) recurrence must be considered ahead of surgery. This study was undertaken to identify pre-operative risk factors for early intrahepatic recurrence of HCC after curative resection in a large-scale.
Materials and Methods
We retrospectively reviewed the preoperative three-phase multi-detector CT (MDCT) and laboratory data for 240 HCC patients who underwent curative resection; tumor size, number, gross shape, capsule integrity, distinctiveness of tumor margin, portal vein thrombosis (PVT), alpha-fetoprotein level (AFP), and protein induced by vitamin K absence-II (PIVKA-II) levels were assessed. Surgical pathology was reviewed; tumor differentiation, capsule, necrosis, and micro-vessel invasion were recorded.
Results
HCC recurred in 61 patients within six months (early recurrence group), but not in 179 patients (control group). In univariate analysis, large tumor size (p = 0.018), shape (p = 0.028), poor capsule integrity (p = 0.046), elevated AFP (p = 0.015), and PIVKA-II (p = 0.008) were significant preoperative risk factors. Among the pathologic features, PVT (p = 0.023), Glisson's capsule penetration (p = 0.033), microvascular invasion (p < 0.001), and poor differentiation (p = 0.001) showed statistical significance. In multivariate analysis, only the histopathologic parameters of microvascular invasion and poor differentiation achieved statistical significance.
Hepatic resection is the mainstay of curative treatment for hepatocellular carcinoma (HCC).1-4 However, debate is ongoing over the best way to select candidates for surgery and how to decide the extent of hepatectomy. The decision of whether or not to proceed with surgery depends mainly on the functional reserve of the liver and the estimated risk of recurrence.3,5-9 Although an ideal limit of residual liver function to serve as a guideline in choosing a specific treatment modality has not yet been established, preoperative measurement of liver function is not difficult. Meanwhile, preoperative estimation of the risk of tumor recurrence remains ambiguous. Therefore, it seems that the stratification of preoperative risk factors for tumor recurrence is important not only in deciding whether or not to proceed with surgical resection, but also in determining the optimal extent of hepatectomy in HCC if surgery is to proceed.
The purpose of this study was to identify risk factors that could be detected preoperatively and postoperatively for early recurrence of HCC after curative resection. For the preoperative decision about hepatic resection, we analyzed dynamic contrast-enhanced CT scans and tumor markers. For post-operative treatment planning, we evaluated surgical histopathologic features.
We retrospectively reviewed the medical records of 414 consecutive patients who received curative resection for HCC between January 2001 and February 2006 in one tertiary medical center. Excluded from the study were patients who either received liver transplantation or lobectomy for palliative treatment (n = 22); patients whose preoperative radiologic evaluation was not performed with a three-phase dynamic enhanced multi-detector CT (MDCT) scan within one month before surgery (n = 119); patients whose tumor pathology was not that of pure hepatocellular carcinoma, for example combined hepatocellular-cholangiocarcinoma (n = 23); and patients who did not complete the six-month follow-up period (n = 10). This process defined a study population of 240 patients, and 181 men and 59 women between 31 and 76 years old (mean ± standard deviation: 53.51 ± 9.04 years) were included. One hundred and one patients had experience of receiving transarterial chemoembolization prior to surgery, while 139 patients had no experience of treatment prior to surgery. The study population was later divided into early recurrence and non-recurrence groups on the basis of six-month follow-up results.
Preoperative CT images were obtained by one of the following commercially available MDCT; 4, 16, and 64 channel MDCT scanners (Somatom Plus 40, Sensation 16, and Sensation 64, Siemens Medical Systems, Erlangen, Germany). Each patient was injected with 120 to 150 mL of iobitridol (Xenetix 300; Guerbet, Aulnay-sous-Bois, France) or iohexol (Omnipaque 300; Daiichi Pharmaceutical, Tokyo, Japan) through an 18-gauge venous cannula placed at the antecubital fossa for contrast injection, using a mechanical injector with a fixed duration of 30 seconds. After unenhanced images were obtained, dynamic three-phase imaging was performed. The hepatic arterial, portal venous, and delayed phases were scanned at 15 seconds, 40 seconds, and 180 seconds, respectively, after the aorta reached 100 HU.
The preoperative CT scan of each patient was reviewed by a gastrointestinal radiologist with 8 years of experience in hepatobiliary imaging. No clinical, laboratory, or pathology information other than the presence of HCC was provided during image analysis.
The tumor's size (the longest diameter on the axial plane), shape, capsule, and margin were evaluated. Tumor shape was classified into four categories: nodular (round in shape with size less than 5 cm), massive expanding (round in shape with size equal to or greater than 5 cm), multinodular confluent, or infiltrative type. Tumor capsule integrity was assessed on a five-point scale according to the percentage of the tumor surface covered by the capsule (grade 1: covering more than 75%, grade 2: covering 51 to 75%, grade 3: covering 26 to 50%, grade 4: covering no more than 25% or absent capsule, and grade 5: could not be evaluated because of complete necrosis). The number of identifiable tumor lesions that appeared to be hyperdense on arterial phase and washed out on equilibrium phase images was counted. Portal vein thrombosis was considered to be present if a filling defect in the portal vein was observed at the portal phase of contrast enhancement.
Serology studies of antigen and antibodies with or without supplemental DNA studies revealed evidence of either active or inactive states of underlying B viral hepatitis (HBV) in 217 patients and C viral hepatitis (HCV) in 14 (one patient had both B and C viral hepatitis). Eight patients did not show evidence of either HBV or HCV hepatitis. Two patients had no records of serology tests for reasons that could not be determined.
Alpha-fetoprotein level (AFP) and protein induced by vitamin K absence-II (PIVKA-II) levels were recorded if blood samples obtained within a month before surgery were available.
The surgical pathology report for each patient was reviewed; and features including the presence or absence of portal tumor thrombi, microscopic vessel invasion, tumor capsule formation, and the success or failure of obtaining a tumor-free surgical resection margin were recorded. The degree of tumor differentiation was categorized according to the Edmondson-Steiner classification as low-grade (Edmondson-Steiner grade I and II) and high-grade tumor (Edmondson-Steiner III and IV). Tumor differentiation could not be assessed in 39 patients because of extensive necrosis of the tumor, and these patients were recorded as having missing data. The extent of tumor necrosis reported by the pathologist was recorded; we considered necrosis of 95% or more of the entire tumor as nearly total necrosis and classified each patient either in the group of nearly total necrosis (≥ 95% necrosis of the whole tumor) or the low necrosis group (< 95% necrosis of whole tumor). The physical relation of the tumor with the Glisson's capsule was categorized according to a three-point scale (grade 1: tumor separated from the Glisson's capsule by normal liver parenchyma, grade 2: direct contact of tumor with the Glisson's capsule without microscopic evidence of tumor penetration, and grade 3: microscopic evidence of tumor penetrating the Glisson's capsule).
Patients were regularly followed up with dynamic CT scans and serum tumor markers (AFP and/or PIVKA-II) at 3 to 6 months after surgery using additional liver MRI, conventional angiography, and/or ultrasound if necessary. Early recurrence was defined as tumor recurrence identified within six months after surgery.
Post-operative HCC recurrence was considered to be present if either a focal lesion was identified measuring at least two centimeters with arterial hypervascularization which was demonstrated in at least two imaging modalities, or a hypervascular nodule exceeding two centimeters was noted in a single imaging study in the presence of over 400 ng/mL AFP.10
In addition, any new nodule that had appeared during the follow-up period, exceeding 2 cm in size, or a newly appearing nodule showing contrast washout leading to hypoattenuation in the equilibrium phase was considered to be highly suspicious for HCC recurrence regardless of size11 and was confirmed with supplemental biopsy or short-term follow-up CT or MRI.
Among the 240 patients who were included in the study, 61 patients were proved to have tumor recurrence within six months. The other 179 patients did not show evidence of tumor recurrence during the same period. No statistically significant difference was found in the age (p = 0.226) and gender (p = 0.085) profile between the early recurrence and non-recurrence groups.
According to univariate analysis for preoperative findings, no significant difference in early recurrence was noted according to the viral pathogen that caused the hepatitis (p = 0.151) or the history of receiving previous trans-arterial chemoembolization (p = 0.616). However, the serum levels of AFP (p = 0.015) and PIVKA-II (p = 0.008) were significantly higher in the early recurrence group.
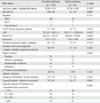
Univariate analysis of the preoperative CT features showed that the shape of the tumor (p = 0.028) (Fig. 1), size (p = 0.018), and capsule integrity (p = 0.046) were related to a statistically significant increase in the risk for early post-operative HCC recurrence. However, the number of tumors (p = 0.118), the number of the liver segments involved with the tumor (p = 0.526), the presence of portal vein thrombosis (p = 0.216), and tumor margins (p = 0.564) did not show statistical significance (Table 1).
Univariate analysis showed that histopathology parameters such as the presence of portal vein tumor thrombi (p = 0.023), microscopic vascular tumor invasion (p < 0.001), physical contact of the Glisson's capsule by the tumor with or without tumor penetration (p = 0.033), failure to achieve tumor cell clear resection margins (p = 0.019), and Edmondson-Steiner grade III and IV (p = 0.001) indicated a higher risk of early post-operative HCC recurrence that was statistically significant. The presence or absence of microscopic capsule formation (p = 0.132) and the presence of nearly total necrosis (p = 0.212) did not show statistical significance (Table 2).
Multivariate analysis with backward logistic regression revealed a statistically significant increase of risk for early post-operative tumor recurrence caused by the presence of microscopic vascular invasion which was proved by histology (odds ratio = 2.557) and Edmondson-Steiner grade III and IV (odds ratio = 2.814) (Table 3).
Intrahepatic tumor recurrence of HCC after treatment has been explained by two different mechanisms, either secondary metastasis or de novo development of a separate primary HCC.12 Chronic viral hepatitis and/or cirrhosis are usually present in HCC patients, and these conditions are risk factors which substantially contribute to the development of HCC.12-14 Therefore, even if a primary HCC is cured, HCC recurrence by newly developed tumors is still more or less inevitable. However, it is very difficult to differentiate intrahepatic metastasis that originated from the primary tumor from newly developed de novo HCC. Imamura, et al.15 suggested that early and late peak recurrence of HCC after resection could roughly represent intrahepatic metastasis and de novo HCC development, respectively. Their suggestion is based on the analysis of the different factors associated with early (non-anatomical resection, presence of micro-vessel invasion, and serum AFP > 32 ng/mL) and late (hepatitis activity, multiple tumors, and gross tumor classification) recurrence. Although the pathogenesis of recurrence is still under investigation, early recurrence has stronger clinical importance compared with late recurrence considering the morbidity and mortality of surgery, cost-effectiveness, and potential benefits of alternative treatments. To our knowledge, however, large scale studies on the evaluation of the radiologic, laboratory and surgical pathological factors associated with early (within six months) recurrence of HCC after curative resection remain limited.
Large tumor size,16 absence of tumor capsule, indistinct margins,17 and elevated serum levels of AFP15,18 have been reported as parameters which increase the risk of early tumor recurrence. In the current study, univariate analysis showed AFP (p = 0.015) and PIVKA-II (0.008) levels to have statistical significance for predicting the risk of HCC recurrence within six months. However, the role of tumor markers seems to be limited, because statistical significance was not reproduced in multivariate analysis.
In the present study, size, gross shape of tumor, and capsule integrity were other radiological findings that showed statistical significance, suggesting higher risk of early tumor recurrence in univariate analysis. However, these factors failed to maintain statistical significance in multivariate analysis.
The presence of portal vein thrombosis identified in preoperative CT scans did not have statistically significant influence in the early HCC recurrence (p = 0.216). This unexpected result could be due either to selection bias or the small number of patients with positive portal vein thrombosis in our study. However, the observation that portal vein thrombosis did not show statistical significance in early HCC recurrence is consistent with a previous study.17
Risk factors reported in previous articles on the basis of surgical pathology are relatively consistent more with each other. The presence of microscopic vascular invasion is a factor repeatedly indicated as a potent risk factor.15,16,18,19 Advanced histopathologic grade is also reported to have statistical significance for HCC recurrence.18,20 Similarly, our study showed microscopic vascular invasion and advanced histopathologic grades to have sustained statistical significance, suggesting a higher risk of early recurrence in multivariate analysis. Other factors, including positive portal vein tumor thrombi in the surgical specimen and direct tumor contact with the Glisson's capsule with or without tumor penetration, were found to be parameters with statistical significance only in univariate analysis.
Previous history of trans-arterial chemoembolization and the presence of nearly total necrosis of tumor with or without previous treatment did not show statistical significance in relation with the rate of early tumor recurrence. In general practice, more advanced tumors tend to receive more intensive preoperative adjuvant therapies; therefore, we believe that selection bias with respect to the degree of preoperative adjuvant therapies received does exist in our study population. In spite of the statistically insignificant results produced by the current study, we do not suggest that preoperative adjuvant therapies are unnecessary.
In conclusion, HCC patients with positive microscopic vascular invasion of tumor and high Edmondson-Steiner grades have a statistically significantly higher risk of early postoperative recurrence. Most parameters of HCC that are detectable preoperatively, including clinical and radiological features, have limited significance in the prediction of postoperative early recurrence.
Figures and Tables
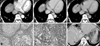 | Fig. 1A 56-year-old female with early recurrent HCC after segmentectomy. The AFP level in a blood sample obtained on the same day as the CT scan was 1076.84 IU/mL. (A) Arterial phase of the preoperative CT obtained by a 4-slice MDCT. A mass measuring approximately 2.2 cm in diameter which was later proven by surgery to be hepatocellular carcinoma is observed at the dome of the liver, presenting as a multinodular confulent nodule (arrow). (B) Early washout (arrow) of contrast of this nodule is observed during the portal venous phase, an enhancement pattern consistent with HCC. (C) The equilibrium phase of the preoperative CT. A linear enhancement structure (black arrow) was noted which was considered to be the radiological capsule. The radiological capsule was assessed to cover less than 25% of the tumor circumference (capsule grade 4). The margin of the nodule is poorly defined (white arrow). (D) Microscopic findings show high grade (Edmondson-Steiner grade III) hepatocellular carcinoma; original magnification, ×200; hematoxylin-eosin (H & E). (E) Microscopic examination revealed frequent microvessel tumor invasion (white arrows), original magnification, ×200; hematoxylin-eosin (H & E). (F) Marked increase of AFP level (10865.27 IU/mL) was observed at the fifth postoperative-month blood test. He underwent a CT scan, which revealed an infiltrative hypervascular mass (white arrow). Another 1 cm sized hypervascular nodule (black arrow) is noted, which increased further in size and measured to be 2.2 cm at a CT scan performed 4 months afterwards, and the findings were highly suggestive of a HCC nodule. HCC, hepatocellular carcinoma; MDCT, multi-detector CT. |
Table 1
Univariate Analysis of Preoperative CT and Laboratory Parameters in Patients with and without Early HCC Recurrence after Lobectomy
References
1. Clark HP, Carson WF, Kavanagh PV, Ho CP, Shen P, Zagoria RJ. Staging and current treatment of hepatocellular carcinoma. Radiographics. 2005. 25:Suppl 1. S3–S23.

2. Ulmer SC. Hepatocellular carcinoma. A concise guide to its status and management. Postgrad Med. 2000. 107:117–124.
3. Ng KK, Vauthey JN, Pawlik TM, Lauwers GY, Regimbeau JM, Belghiti J, et al. Is hepatic resection for large or multinodular hepatocellular carcinoma justified? Results from a multi-institutional database. Ann Surg Oncol. 2005. 12:364–373.

4. Mor E, Kaspa RT, Sheiner P, Schwartz M. Treatment of hepatocellular carcinoma associated with cirrhosis in the era of liver transplantation. Ann Intern Med. 1998. 129:643–653.

5. Bruix J, Llovet JM. Prognostic prediction and treatment strategy in hepatocellular carcinoma. Hepatology. 2002. 35:519–524.

6. Fong Y, Sun RL, Jarnagin W, Blumgart LH. An analysis of 412 cases of hepatocellular carcinoma at a Western center. Ann Surg. 1999. 229:790–799.

7. Yamamoto J, Okada S, Shimada K, Okusaka T, Yamasaki S, Ueno H, et al. Treatment strategy for small hepatocellular carcinoma: comparison of long-term results after percutaneous ethanol injection therapy and surgical resection. Hepatology. 2001. 34:707–713.

8. Poon RT, Fan ST, Wong J. Selection criteria for hepatic resection in patients with large hepatocellular carcinoma larger than 10 cm in diameter. J Am Coll Surg. 2002. 194:592–602.

9. Hanazaki K, Kajikawa S, Shimozawa N, Shimada K, Hiraguri M, Koide N, et al. Hepatic resection for hepatocellular carcinoma in diameter of > or = 10 cm. Hepatogastroenterology. 2002. 49:518–523.
10. Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, et al. Clinical management of hepatocellular carcinoma Conclusions of the Barcelona-2000 EASL conference European Association for the Study of the Liver. J Hepatol. 2001. 35:421–430.

11. Bolondi L, Gaiani S, Celli N, Golfieri R, Grigioni WF, Leoni S, et al. Characterization of small nodules in cirrhosis by assessment of vascularity: the problem of hypovascular hepatocellular carcinoma. Hepatology. 2005. 42:27–34.

12. Yamamoto M, Matsuda M, Iimuro Y, Fujii H, Nagahori K, Ainota T. Intrahepatic distant metastasis and metachronous multicentric occurrence in solitary hepatocellular carcinoma of less than five centimeters in diameter. Surg Today. 1993. 23:969–978.

13. Colombo M, de Franchis R, Del Ninno E, Sangiovanni A, De Fazio C, Tommasini M, et al. Hepatocellular carcinoma in Italian patients with cirrhosis. N Engl J Med. 1991. 325:675–680.

14. Tsukuma H, Hiyama T, Tanaka S, Nakao M, Yabuuchi T, Kitamura T, et al. Risk factors for hepatocellular carcinoma among patients with chronic liver disease. N Engl J Med. 1993. 328:1797–1801.

15. Imamura H, Matsuyama Y, Tanaka E, Ohkubo T, Hasegawa K, Miyagawa S, et al. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol. 2003. 38:200–207.

16. Shirabe K, Kanematsu T, Matsumata T, Adachi E, Akazawa K, Sugimachi K. Factors linked to early recurrence of small hepatocellular carcinoma after hepatectomy: univariate and multivariate analyses. Hepatology. 1991. 14:802–805.

17. Lim JH, Jang HJ, Kim EY, Park CK, Joh JW, Kim YI. Early recurring hepatocellular carcinoma after partial hepatic resection: preoperative CT findings. Korean J Radiol. 2000. 1:38–42.

18. Shah SA, Cleary SP, Wei AC, Yang I, Taylor BR, Hemming AW, et al. Recurrence after liver resection for hepatocellular carcinoma: risk factors, treatment, and outcomes. Surgery. 2007. 141:330–339.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download