Abstract
Purpose
In the present study, we tested whether the presence of metabolic syndrome (MetS) would worsen the features of inflammation, plasma omega 3 fatty acid levels and antioxidant potential in treated hypertensive patients.
Materials and Methods
Two groups were classified by the components of MetS: a reference group of treated hypertensive subjects: hypertension (HTN) group (n = 39) and with more than two additional MetS components: HTN with Mets group (n = 40). We further compared the parameters between HTN group and HTN with MetS group.
Results
The results showed that age (p < 0.001) and body mass index (BMI) (p < 0.001) were significantly different between HTN group and HTN with MetS group. Age- and BMI-adjusted total radical trapping antioxidant potential (TRAP) (p < 0.01) was significantly lower, whereas age- and BMI-adjusted CD (p < 0.05) and interleukin (IL) 6 (p < 0.05) were significantly higher in HTN with MetS group than in HTN group. Moreover, HTN with MetS group had significantly lower levels of age- and BMI-adjusted plasma phospholipid eicosapentaenoic acid (EPA) than HTN group (p < 0.05). On the other hand, the levels of age- and BMI-adjusted intracellular cell adhesion molecule-1 (ICAM-1), adiponectin and high molecular weight (HMW)-adiponectin were not significantly different between the groups.
The importance of the metabolic syndrome (MetS) in the pathogenesis of cardiovascular disease has increasingly been addressed. Recently, accumulating epidemiological and clinical evidence has shown that MetS is associated with increased risks for cardiovascular disease1,2 and type 2 diabetes mellitus.3,4 More recently, MetS was found to be an independent predictor for cardiovascular disease in hypertensive subjects.5 Furthermore, several subsequent studies reported that MetS was associated with the hypertension-related target organ damage6-8 such as left ventricular hypertrophy and impaired arterial distensibility, suggesting MetS with subclinical organ damage as a mediator of enhanced cardiovascular risks.
The mechanisms underlying the association of MetS in hypertension with the development of cardiovascular disease remain uncertain. However, several pathways which are mediated by endothelial dysfunction,9 arterial stiffness5,10 and inflammation may be involved.11 Given that hypertension, as a primary part of MetS, is usually accompanied by other cardiometabolic risk factors,12 it is possible that the cardiovascular risk profiles are greatly exaggerated in the presence of underlying MetS in hypertension, thus possibly leading to increased risks for cardiovascular morbidity and mortality.
In the present study, we hypothesized that the presence of MetS would result in unfavorable patterns for cardiovascular risk profiles in treated hypertensive patients. To test this possibility, we evaluated the inflammatory markers and antioxidant potential together with blood omega 3 fatty acid compositions which have emerged as a risk factor for cardiovascular disease in hypertension with- or without MetS.
One hundred thirty-five hypertensive patients who had been diagnosed and treated at Yonsei Cardiovascular Center participated in the present study. Subjects with any of the following conditions were excluded from participation: valvular heart disease, peripheral vascular disease, significant systemic disease, history of inflammatory disease and/or on anti-inflammatory medications, patient taking aldosterone antagonists at the time of study enrollment, clinically significant atrioventricular conduction disturbance, history of atrial fibrillation or other serious arrhythmia, severe hypertension (> 210/130 mmHg) or serum creatinine greater than 1.4 mg/dL. They underwent a brief physical examination for measurement of seated blood pressure using a sphygmomanometer with an appropriate cuff. Two measurements were taken at least five minutes apart and the mean value was used for analysis. Weight was measured using a standard balance beam scale or an electronic scale and height was measured using a height rod of a standard beam scale, or a wall-mounted stadiometer. Body Mass Index (BMI) was calculated as weight in kg divided by height in meters squared. Waist circumference was measured twice to the nearest 0.1 cm with a flexible tape measure at the level of the minimum circumference, usually at the level of the navel. All patients gave written informed consent, and the Institutional Review Board at the Yonsei University Medical Center approved the study protocol.
MetS was defined as three or more of the following abnormalities according to modified NCEP ATP III definition (ATP III criteria and the WHO Western Pacific Region obesity criteria)13,14: 1) abdominal obesity, waist circumference ≥ 90 cm in men, ≥ 80 cm in women; 2) hypertriglyceridemia, ≥ 150 mg/dL; 3) high-density lipoprotein (HDL)-cholesterol, < 40 mg/dL in men and < 50 mg/dL in women; 4) hypertension (systolic blood pressure ≥ 130 mmHg or diastolic pressure ≥ 85 mmHg) or on anti-hypertensive medication; 5) high fasting glucose, ≥ 110 mg/dL or under treatment for diabetes.
Fasting blood samples were taken, and serum cholesterol, low-density lipoprotein (LDL)-cholesterol and HDL-cholesterol were measured by enzymatic methods with commercially available kits (Choongwae, Seoul, Korea). Serum triglyceride levels were analyzed using a total glycerol test kit (Roche, Basel, Switzerland). All determinants were done on a Hitachi 747 auto-analyzer (Hitachi Ltd., Tokyo, Japan). Fasting serum glucose concentrations were measured by the glucose oxidase method using a Beckman Glucose Analyzer (Beckman Instruments, Irvine, CA, USA). The fasting serum insulin level was measured with an immunoradiometric assay and a gamma counter (Hewlett Packard, Meriden, CT, USA). We calculated homeostasis model of insulin resistance (HOMA-IR) using the equation HOMA-IR = fasting insulin (µU/mL) × glucose (mmol/L)/22.5.15
Baseline LDL conjugated dienes (CD) were determined according to Ahotupa, et al.16 with little modification. One hundred µL of plasma was added to 700 µL of heparin citrate buffer (0.064 M trisodium citrate, 50,000 IU/L heparin, pH 5.05), and the suspension was allowed to stand for 10 min at room temperature. The insoluble lipoproteins were then sedimented by centrifugation at 1,000 g for 10 min. The pellet was resuspended in 100 µl of 0.1 M Naphosphate buffer containing 0.9% NaCl (pH 7.4). Lipids were extracted from 100 µL of LDL suspension by chloroform-methanol (2 : 1), dried under nitrogen, then redissolved in cyclohexane and analyzed spectrophotometrically at 234 nm. Oxidation during the sample preparation was prevented by adding ethylenediaminetetraacetic acid (EDTA).
TRAP was measured by a modification of the photometric method according to Rice-Evans and Miller.17 The method for measuring antioxidant activity is based on the antioxidants' inhibition of absorbance of the radical cation, 2,2'-azinobis (3-ethylbenzothiazoline 6-sulfonate) (ABTS+). The ABTS+ radical cation was formed by the interaction of ABTS+ (150 µM) with ferrylmyoglobin radical species, generated by the activation of metmyoglobin (2.5 µM) with H2O2 (75 µM). Ten µL of sample/buffer/Trolox-standard were added to tubes containing 400 µL of phosphate buffered saline (PBS) buffer, 20 µL of metmyoglobin and 400 µL of ABTS, and the contents were mixed by vortexing. The reaction was initiated by addition of 170 µL of H2O2. Absorbance was measured with a spectrophotometer at 734 nm after 6 min of incubation. Values were expressed as trolox equivalent antioxidant capacity (TEAC), which is defined as mM concentration of Trolox antioxidant capacity, determined by using a calibration curve.
Total lipids were extracted according to the method of Folch, et al., and the phospholipid fraction was isolated by using thin-layer chromatography with hexane : diethyl ether : acetic acid (80 : 20 : 2) development solvent. The phospholipid fractions were then directly transesterified to prepare fatty acid methyl esters (FAMEs) by the method of Lepage and Roy.18 The FAME of individual fatty acids of phospholipids was separated on a gas chromatograph (model 6890, Agilent Technologies Inc, Palo Alto, CA, USA) equipped with a capillary column (SP-2560; 100m, Supelco, Bellefonte, PA, USA) as previously described.19 Peak retention times were identified by comparison with known standard (37 component FAME mix, Supelco, Bellefonte, PA; GLC37, NuCheck Prep, Elysian, MN, USA) and analyzed with the ChemStation software (Agilent Technologies). Plasma phospholipid levels of EPA and DHA were expressed as the percentage of total fatty acids.
Plasma interleukin-6 (IL-6, R&D Systems, Minneapolis, MN, USA), intercellular cell adhesion molecule-1 (ICAM-1, R&D Systems, Minneapolis, MN, USA), adiponectin (Linco Research, St. Charles, MO, USA) and high molecular weight adiponectin (HMW-adiponetin, Linco Research, St. Charles, MO, USA) were measured using an enzyme-linked immunoassay according to manufacturer's instructions.
The Statistical Package for Social Science (SPSS; SPSS Inc, Chicago, IL, USA) 12.0 software package was used for statistical analysis. Data are presented as mean ± S.E. Each variable was examined for normal distribution, and abnormally distributed variables were log-transformed. Frequency distributions were tested by χ2 test among the groups. General Linear Model (GLM) was used to test the differences of parameters between the groups after adjusting age and BMI. p values < 0.05 were considered statistically significant.
Of the 135 treated hypertensive subjects, 124 subjects were available for the classification of MetS and further analyzed for this study. Based on the criteria of MetS, two groups were classified by the components of MetS: a reference group of treated hypertensive subjects: HTN group (n = 39) and with more than two additional MetS components: HTN with Mets group (n = 40), and we further compared the parameters between HTN group and HTN with MetS group. The proportions of patients taking these medications were similar between the groups (Table 1).
Table 1 describes baseline characteristics and clinical parameters between HTN group and HTN with MetS group. Age (p < 0.001) and BMI (p < 0.001) were significantly different between the groups. Systolic and diastolic blood pressure and gender distribution were not significantly different. As expected, serum concentrations of TG (p < 0.001) and HDL-cholesterol (p < 0.001) and the levels of HOMA-IR (p < 0.001) were significantly different between the groups. On the other hand, serum concentrations of total cholesterol and LDL-cholesterol were similar between the two groups.
Table 2 compares LDL-CD, TRAP, phospholipid DHA and inflammatory markers between the two groups. Because age and BMI were significantly different between the groups, we used the age- and BMI-adjusted values for all analyses. The results showed that age- and BMI-adjusted TRAP (p < 0.01) was significantly lower, whereas age- and BMI-adjusted CD (p < 0.05) was significantly higher in HTN with MetS group than in HTN group. On the other hand, the levels of age- and BMI-adjusted ICAM-1, adiponectin and HMW-adiponectin were not significantly different between the groups.
Moreover, HTN with MetS group had significantly lower levels of age- and BMI-adjusted plasma phospholipid EPA (p < 0.05) and significantly higher levels of age- and BMI-adjusted IL-6 (p < 0.05) than in HTN group, whereas no differences were found in DHA between the two groups (Fig. 1).
To examine the synergistic effects of MetS in hypertension on cardiovascular risk profiles, we evaluated plasma LDL-CD as a marker for lipid peroxidation, TRAP as a reflection of antioxidant potential, inflammation (IL-6, ICAM-1, total- and HMW-adiponectin) and plasma phospholipid omega 3 fatty acid contents. Our results showed that the levels of LDL-CD and IL-6 were increased whereas the levels of TRAP and plasma phospholipid EPA were decreased in hypertensive patients with MetS compared to those in hypertensive patients.
It is well demonstrated that increased oxidative stress underlies the pathophysiology of hypertension by directly influencing vascular wall cells20 and oxidative stress has been associated with the onset of cardiovascular complications in subjects with the metabolic syndrome.21 Several observational and experimental studies showed impaired antioxidant systems with decreases in antioxidant capacity and increases in lipid peroxidation in MetS,22 which is consistent with our results. Hyperglycemia, a key component of the MetS, might trigger oxidative stress by several mechanisms either independently or associated with other conditions.23 It includes glucose auto-oxidation, advanced glycated end product (AGE) formation, abnormal arachidonic acid metabolism and its coupling to cyclooxygenase catalysis.23 The enhanced lipid peroxidation and reduced antioxidant potential in hypertension with MetS, shown in the present study, can be explained, in part, through the mechanisms mentioned above. Considering the atherogenic effect of oxidative alterations in the atherosclerotic process,24 our results provide a possible mechanism which links MetS in hypertension to increased cardiovascular risks.
Inflammation has been established to be a major mediator of increased cardiovascular risks.25 As evidenced by elevations of circulating levels of C-reactive protein (CRP) and several inflammatory cytokines including IL-6 and tumor necrosis factor-α (TNF-α), chronic inflammation has been associated with hypertension and MetS.26,27 Consistent with the earlier findings,26,27 we observed in the present study that plasma levels of IL-6 were significantly higher in the hypertensive patients with MetS. Adiponectin, a most abundant adipokine, is primarily released from adipose tissue, and adipose tissue is believed to be an important mediator of oxidative stress and inflammation.28 The profound metabolic effects of adiponectin on glucose and lipid metabolism provide an evidence to link obesity-related chronic inflammation and atherosclerosis.28,29 HMW-adiponectin is a multimeric form of adipoenctin and has been suggested as a more sensitive biomarker for insulin resistance and abdominal adiposity than total adiponectin.29 In contrast to the previous studies,28-31 there were no significant differences in total- and HMW-adiponectin levels between the groups. This may be attributed, in part, to the study population with narrow range in BMI (mean 24.6 kg/m2, 20.0-29.8) in the present study. However, we found gender-specific differences in total- and HMW adiponectin between hypertension group and hypertension with MetS group. In females, plasma levels of total- and HMW adiponectin were significantly lower in hypertension with MetS group than hypertension group, but not in males (data not shown). Also, the effect of MetS in hypertension on the levels of TRAP was mainly derived from females (data not shown). Our results partly support that MetS may have different relative importance for cardiovascular disease according to gender, and that the prognostic impact of MetS is better in females than in males.2,6,32
Emerging evidence showed that omega-3 fatty acids (EPA and DHA) have a variety of beneficial effects on blood pressure, platelet aggregation, inflammatory responses and vasodilation as well as lipid and lipoprotein metabolism.33 Furthermore, omega 3 fatty acids were found to protect cardiovascular morbidity and mortality,34,35 suggesting as an emerging risk factor for cardiovascular disease. The mechanisms by which omega 3 fatty acids exhibit cardioprotective effects include that they alter membrane physical characteristics and the activity of membrane-bound proteins.36 Furthermore, they can interact with ion channels and also act as ligands for several nuclear transcription factors.36 The major finding in this study was that plasma phospholipid EPA content was significantly lower in hypertensive patients with MetS than that in hypertensive patients, whereas no difference was observed in DHA. Given that EPA is known to inhibit the production of proin-flammatory eicosanoids more efficiently than DHA,37 it may modulate inflammatory conditions, oxidative stress accompanied by reduced antioxidant potential, which was not experimentally proved in this study. However, only plasma phosholipid EPA, but not DHA, was slightly reduced, therefore, it is also possible to consider potential metabolic alterations. EPA may in part originate from retroconversion of DHA in the peroxisome which requires partial β-oxidation.38 Thus, diminished levels of EPA and concomitantly enhanced DHA may occur under conditions of reduced peroxisomal β-oxidation in the MetS. Further experimental studies are needed to elucidate the mechanism for these relationships described in this study.
The main limitations of this study are small sample size and cross-sectional setting, thus making it hard to draw causality. Also, we could not exclude the possibility that lack of nutritional information on fatty acids and fish consumption might confound the results. Nevertheless, our results extended several previous studies by showing synergistic effects of MetS in hypertension on inflammation and oxidative stress. Furthermore, we found decreased plasma phospholipid EPA in hypertensive patients with MetS, suggesting possible impact of MetS on inflammatory response in hypertension.
To conclude, our results showed increased inflammatory marker, reduced plasma phospholipid EPA content and reduced antioxidant potential in treated hypertensive patients in the presence of MetS. Considering that blood and tissue omega 3 fatty acids are the reflections of dietary intakes,36 our results suggest the importance of changes of therapeutic lifestyle to modify the features of MetS. Intensive nutritional program needs to be encouraged in these hypertensive patients with MetS in clinical settings.
Figures and Tables
Fig. 1
Comparison of age- and BMI-adjusted levels of IL-6 and EPA between hypertension group and hypertension with MetS group (*p < 0.05). BMI, body mass index; IL-6, interleukin-6; EPA, eicosapentaenoic acid; MetS, metabolic syndrome.

Table 1
Baseline Characteristics and Serum Lipids and HOMA-IR of Subjects according to the Presence of MetS
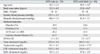
Table 2
Age- and BMI-adjusted Lipid Peroxidation, Inflammatory Markers and Plasma DHA Contents of Subjects according to the Presence of MetS

BMI, body mass index; DHA, docosahexaenoic acid; MetS, metabolic syndrome; HTN, hypertension; LDL-CD, conjugated dienes in low-density lipoprotein; TRAP, total radical trapping antioxidant potential; HMW, high molecular weight adiponectin.
Values are Mean ± S.E. All data presented are adjusted for age and BMI.
*p < 0.05.
†p < 0.01.
ACKNOWLEDGEMENTS
This work was supported by the Korea Science and Engineering Foundation (KOSEF) grant funded by the Korea government (MOST) (R01-2008-000-20879-0).
References
1. Isomaa B, Almgren P, Tuomi T, Forsén B, Lahti K, Nissén M, et al. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care. 2001. 24:683–689.

2. McNeill AM, Rosamond WD, Girman CJ, Golden SH, Schmidt MI, East HE, et al. The metabolic syndrome and 11-year risk of incident cardiovascular disease in the atherosclerosis risk in communities study. Diabetes Care. 2005. 28:385–390.

3. Ford ES, Li C, Sattar N. Metabolic syndrome and incident diabetes: current state of the evidence. Diabetes Care. 2008. 31:1898–1904.
4. Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005. 112:2735–2752.
5. Schillaci G, Pirro M, Vaudo G, Gemelli F, Marchesi S, Porcellati C, et al. Prognostic value of the metabolic syndrome in essential hypertension. J Am Coll Cardiol. 2004. 43:1817–1822.

6. Schillaci G, Pirro M, Pucci G, Mannarino MR, Gemelli F, Siepi D, et al. Different impact of the metabolic syndrome on left ventricular structure and function in hypertensive men and Women. Hypertension. 2006. 47:881–886.

7. Cuspidi C, Meani S, Fusi V, Severgnini B, Valerio C, Catini E, et al. Metabolic syndrome and target organ damage in untreated essential hypertensives. J Hypertens. 2004. 22:1991–1998.
8. Leoncini G, Ratto E, Viazzi F, Vaccaro V, Parodi D, Parodi A, et al. Metabolic syndrome is associated with early signs of organ damage in nondiabetic, hypertensive patients. J Intern Med. 2005. 257:454–460.

9. Lteif AA, Han K, Mather KJ. Obesity, insulin resistance, and the metabolic syndrome: determinants of endothelial dysfunction in whites and blacks. Circulation. 2005. 112:32–38.

10. Schiffrin EL. Vascular stiffening and arterial compliance. Implications for systolic blood pressure. Am J Hypertens. 2004. 17:39S–48S.
11. Rutter MK, Meigs JB, Sullivan LM, D'Agostino RB Sr, Wilson PW. C-reactive protein, the metabolic syndrome, and prediction of cardiovascular events in the Framingham Offspring Study. Circulation. 2004. 110:380–385.

12. Aizawa Y, Watanabe H, Ramadan MM, Usuda Y, Watanabe T, Sasaki S. Clustering trend of components of metabolic syndrome. Int J Cardiol. 2007. 121:117–118.

13. Expert Panel on Detection Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001. 285:2486–2497.
14. Steering Committee of the WHO Western Pacific Region, IASO & IOTF. The Asia-Pacific perspective: redefining obesity and its treatment. 2000. Australia:
15. Mattews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985. 28:412–419.

16. Ahotupa M, Marniemi J, Lehtimäki T, Talvinen K, Raitakari OT, Vasankari T, et al. Baseline diene conjugation in LDL lipids as a direct measure of in vivo LDL oxidation. Clin Biochem. 1998. 31:257–261.

17. Rice-Evans C, Miller NJ. Total antioxidant status in plasma and body fluids; in Methods in Enzymology. 1994. New York: Academic Press;279–293.
18. Lepage G, Roy CC. Direct transesterification of all classes of lipids in a one-step reaction. J Lipid Res. 1986. 27:114–120.

19. Lemaitre RN, King IB, Mozaffarian D, Sotoodehnia N, Rea TD, Kuller LH, et al. Plasma phospholipid trans fatty acids, fatal ischemic heart disease, and sudden cardiac death in older adults: the cardiovascular health study. Circulation. 2006. 114:209–215.

20. Nakazono K, Watanabe N, Matsuno K, Sasaki J, Sato T, Inoue M. Does superoxide underlie the pathogenesis of hypertension? Proc Natl Acad Sci U S A. 1991. 88:10045–10048.

21. Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004. 114:1752–1761.

22. Van Guilder GP, Hoetzer GL, Greiner JJ, Stauffer BL, Desouza CA. Influence of metabolic syndrome on biomarkers of oxidative stress and inflammation in obese adults. Obesity (Silver Spring). 2006. 14:2127–2131.

23. Grattagliano I, Palmieri VO, Portincasa P, Moschetta A, Palasciano G. Oxidative stress-induced risk factors associated with the metabolic syndrome: a unifying hypothesis. J Nutr Biochem. 2008. 19:491–504.

24. Toshima S, Hasegawa A, Kurabayashi M, Itabe H, Takano T, Sugano J, et al. Circulating oxidized low density lipoprotein levels. A biochemical risk marker for coronary heart disease. Arterioscler Thromb Vasc Biol. 2000. 20:2243–2247.
25. Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005. 352:1685–1695.

26. Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest. 1995. 95:2409–2415.

27. Kressel G, Trunz B, Bub A, Hülsmann O, Wolters M, Lichtinghagen R, et al. Systemic and vascular markers of inflammation in relation to metabolic syndrome and insulin resistance in adults with elevated atherosclerosis risk. Atherosclerosis. 2009. 202:263–271.

28. Hotta K, Funahashi T, Bodkin NL, Ortmeyer HK, Arita Y, Hansen BC, et al. Circulating concentrations of the adipocyte protein adiponectin are decreased in parallel with reduced insulin sensitivity during the progression to type 2 diabetes in rhesus monkeys. Diabetes. 2001. 50:1126–1133.

29. Lara-Castro C, Luo N, Wallace P, Klein RL, Garvey WT. Adiponectin multimeric complexes and the metabolic syndrome trait cluster. Diabetes. 2006. 55:249–259.

30. Aso Y, Yamamoto R, Wakabayashi S, Uchida T, Takayanagi K, Takebayashi K, et al. Comparison of serum high-molecular weight (HMW) adiponectin with total adiponectin concentrations in type 2 diabetic patients with coronary artery disease using a novel enzyme-linked immunosorbent assay to detect HMW adiponectin. Diabetes. 2006. 55:1954–1960.

31. Seino Y, Hirose H, Saito I, Itoh H. High molecular weight multimer form of adiponectin as a useful marker to evaluate insulin resistance and metabolic syndrome in Japanese men. Metabolism. 2007. 56:1493–1499.

32. Hunt KJ, Resendez RG, Williams K, Haffner SM, Stern MP. San Antonio Heart Study. National Cholesterol Education Program versus World Health Organization metabolic syndrome in relation to all-cause and cardiovascular mortality in the San Antonio Heart Study. Circulation. 2004. 110:1251–1257.

33. Jacobson TA. Secondary prevention of coronary artery disease with omega-3 fatty acids. Am J Cardiol. 2006. 98:61i–70i.

34. Lemaitre RN, King IB, Mozaffarian D, Kuller LH, Tracy RP, Siscovick DS. n-3 Polyunsaturated fatty acids, fatal ischemic heart disease, and nonfatal myocardial infarction in older adults: the Cardiovascular Health Study. Am J Clin Nutr. 2003. 77:319–325.

35. Hu FB, Bronner L, Willett WC, Stampfer MJ, Rexrode KM, Albert CM, et al. Fish and omega-3 fatty acid intake and risk of coronary heart disease in women. JAMA. 2002. 287:1815–1821.

36. Harris WS. The omega-3 index as a risk factor for coronary heart disease. Am J Clin Nutr. 2008. 87:1997S–2002S.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download