Abstract
We report a rare case of petroclival craniopharyngioma with no connection to the sellar or suprasellar region. MRI and CT images revealed a homogenously enhancing retroclival solid mass with aggressive skull base destruction, mimicking chordoma or aggressive sarcoma. However, there was no calcification or cystic change found in the mass. Here, we report the clinical features and radiographic investigation of this uncommon craniopharyngioma arising primarily in the petroclival region.
Craniopharyngiomas constitute approximately 2 to 4% of all primary intracranial tumors.1 These tumors characteristically arise in the sellar/suprasellar region, although there have been reports of tumor extension away from the sella or recurrences in distant sites after the removal of the primary lesions.2,3 We describe an unusual case of aggressive craniopharyngioma primarily arising from clivus with extention to the posterior fossa that presented with multiple lower cranial nerve palsies.
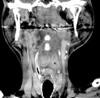
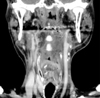
A 55-year-old man with a previous diagnosis of chronic otomastoiditis in his left ear was admitted to our hospital with complaints of a 1-month history of progressive posterior cervicalgia. He also presented with dysphasia and repetitive aspirations three months before his admission. His neurologic examination revealed diffuse paravertebral tenderness, neck motion limitation to the left side, left facial nerve palsy (House-Brackmann grade 4) and severe ipsilateral hearing loss. The findings of a cerebellar function test were unremarkable. There was no previous history of surgical operation. Due to the progressive and severe nature of his symptoms, he underwent magnetic resonance imaging (MRI) of the brain with a 1.5T MR unit. MRI imaging disclosed a homogenously enhancing solid mass occupying the clivus, extending posterolaterally into the epidural space of posterior fossa beyond temporal bone, and anteriorly to prevertebral space at cervicomedullary junction (Fig. 1). Its intracranial portion occupying the posterior fossa engulfed intraosseous segment of left intra cranial artery (ICA). There was no connection between the mass and the sellar or suprasellar regions. The size and location of the pituitary gland were unremarkable. It was mainly isointense to the brain cortex on both T1-weighted and T2-weighted images (Fig. 1). On high resolution bone algorithm CT images revealed that permeative bone destruction was excessively demonstrated in clivus, occipital condyle, petrous and mastoid portions of left temporal bone (Fig. 2). However, there was no calcification or cystic change observed within the lesion. Carotid and vertebral angiography revealed that the tumor was avascular and moved left vertebral artery toward the medial direction (Fig. 2). The differential diagnosis included chordoma, sarcoma and metastasis of skull base.
The patient underwent resection of the mass via suboccipital craniectomy using the left transcondylar approach and C1 hemilaminectomy. The tumor mass began to bulge out when the occipital condyle was drilled. The intracranial segment of the vertebral artery was displaced posteriorly and medially by the tumor mass. The vertebral artery adhered to the surrounding soft tissue and tumor mass itself. The glossopharyngeal, vagal and hypoglossal nerves were intermingled with the tumor mass, which was rubbery and of hard consistency and moderate vascularity. A dural incision was made after the tumor mass was partially removed, but there were no signs of a definite tumor in the intracranial space. The pathological results demonstrated irregular nests of multistratified squamous cells with peripheral palisading of the nuclei and keratin (Fig. 3).
His symptoms, however, were not improved. Then, the patient was referred for radiotherapy at a dose of 72 Gy (40 fractions), however, the radiation therapy was stopped after the administration of the 36 Gy dose because of deterioration of his general condition. One 1 month later, he died of aggravation of aspiration pneumonia which did not respond to antibiotic treatment.
Craniopharyngiomas arise from squamous epithelial rests along the remnants of Rathke's pouch.1 Embryonic Rathke's pouch remnants are frequently observed in the pars tuberalis,4 the posterior wall of the pharynx,5 and the sphenoid bone.6 These patterns of localization explain the frequent occurrence of craniopharyngiomas in the suprasellar region1 and the potential development of craniopharyngiomas in the sphenoid bone6 or nasopharynx.7,8 Other ectopic locations include the pineal gland,9 third ventricle,10 ethmoid sinus,11 and posterior fossa.12-15
Because craniopharyngiomas can extend to the anterior (2-5% of cases), middle (2%), or posterior (1-4%) cranial fossa and infrasellar extension is seen in about 5% of all cases,16 a purely ectopic occurrence showing no sellar extension is very rare. Based on the radiological findings, we concluded that the patient's craniopharyngioma arose from the clivus and was entirely isolated from the sella or suprasellar regions, in agreement with previous reports of a few similar cases.13,15 A possible explanation for the ectopic location of the tumor is the migration of squamous epithelial cell remnants of the obliterated craniopharyngeal canal.
In contrast to the classic radiographic appearance of suprasellar craniopharyngiomas, which usually manifest as cystic or calcified tumors with a spectrum of presenting signs and symptoms, including visual changes, endocrine abnormalities and increased intracranial pressure,17 neither calcification nor cystic components were found in this case. Moreover, the consideration of extensive bone destruction in the clivus, temporal petrous and occipital condyle resulted in the preoperative radiologic diagnosis of chordoma, aggressive sarcoma or metastasis instead of craniopharyngioma.
In summary, although our case did not show any specific radiographic findings to allow for differential diagnosis of craniopharyngioma, the clinical manifestations and radiographic features of the mass suggest that craniopharyngioma should be included in the differential diagnosis of petroclival mass which causes aggressive skull base destruction.
Figures and Tables
Fig. 1
Axial T2-weighted (upper left), T1-weighted (upper center), contrast-enhanced T1-weighted axial (Upper right), coronal (lower left), and sagittal (lower center, right) MR images show a large well-enhancing solid mass occupying the petroclival region with intracranial extension. It dose not show sellar or suprasellar extension. Compared with the brain cortex, it is isointense on both T1-weighted and T2-weighted images.

Fig. 2
Bone-algorithm axial and coronal CT images reveal an extensive, permeative destruction, especially affecting temporal petromastoid and occipital condyle. Left carotid bony canal and jugular foramen were also destroyed. Sclerotic change of left temporal bone might be caused by chronic otomastoiditis. Left vertebral artery is medially displaced by the tumor (lower right) on angiogram.
References
1. McLendon RE, Rosenblum MK, Bigner DD. Russell and Rubinstein's pathology of tumors of the nervous system. 1998. 6th ed. London: A Hodder Arnold Publication.
2. Ragoowansi AT, Piepgras DG. Postoperative ectopic craniopharyngioma. Case report. J Neurosurg. 1991. 74:653–655.
3. Tomita S, Mendoza ND, Symon L. Recurrent craniopharyngioma in the posterior fossa. Br J Neurosurg. 1992. 6:587–590.

4. Goldberg GM, Eshbaugh DE. Squamous cell nests of the pituitary gland as related to the origin of craniopharyngiomas. A study of their presence in the newborn and infants up to age four. Arch Pathol. 1960. 70:293–299.
5. McGrath P. Aspects of the human pharyngeal hypophysis in normal and anencephalic fetuses and neonates and their possible significance in the mechanism of its control. J Anat. 1978. 127:65–81.
6. Cooper PR, Ransohoff J. Craniopharyngioma originating in the sphenoid bone. Case report. J Neurosurg. 1972. 36:102–106.
7. Benitez WI, Sartor KJ, Angtuaco EJ. Craniopharyngioma presenting as a nasopharyngeal mass: CT and MR findings. J Comput Assist Tomogr. 1988. 12:1068–1072.
8. Kanungo N, Just N, Black M, Mohr G, Glikstein R, Rochon L. Nasopharyngeal craniopharyngioma in an unusual location. AJNR Am J Neuroradiol. 1995. 16:1372–1374.
9. Solarski A, Panke ES, Panke TW. Craniopharyngioma in the pineal gland. Arch Pathol Lab Med. 1978. 102:490–491.
10. Linden CN, Martinez CR, Gonzalvo AA, Cahill DW. Intrinsic third ventricle craniopharyngioma: CT and MR findings. J Comput Assist Tomogr. 1989. 13:362–363.
11. Jiang RS, Wu CY, Jan YJ, Hsu CY. Primary ethmoid sinus craniopharyngioma: a case report. J Laryngol Otol. 1998. 112:403–405.
12. Altinörs N, Senveli E, Erdoğan A, Arda N, Pak I. Craniopharyngioma of the cerebellopontine angle. Case report. J Neurosurg. 1984. 60:842–844.
13. Bashir EM, Lewis PD, Edwards MR. Posterior fast craniopharyngioma. Br J Neurosurg. 1996. 10:613–615.
14. Gökalp HZ, Egemen N, Ildan F, Bacaci K. Craniopharyngioma of the posterior fossa. Neurosurgery. 1991. 29:446–448.

15. Link MJ, Driscoll CL, Giannini C. Isolated, giant cerebellopontine angle craniopharyngioma in a patient with Gardner syndrome: case report. Neurosurgery. 2002. 51:221–225. discussion 225-6.

16. Sener RN. Giant craniopharyngioma extending to the anterior cranial fossa and nasopharynx. AJR Am J Roentgenol. 1994. 162:441–442.

17. Eldevik OP, Blaivas M, Gabrielsen TO, Hald JK, Chandler WF. Craniopharyngioma: radiologic and histologic findings and recurrence. AJNR Am J Neuroradiol. 1996. 17:1427–1439.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download