Abstract
Recurrent syncope as a complication of recurrent neck malignancy is an uncommon but well documented association. The syncope is presumed to occur when a tumor mass invades the baroreceptor within the carotid sinus or when it disrupts the afferent nerve fibers of the glossopharyngeal nerve. A 59-year-old man presented with recurrent syncope and headache. He had a wide local excision including tonsillectomy and modified left radical neck dissection for tonsilar cancer 4 years ago. A computed tomography scan revealed ill-defined lesions in left parapharyngeal, carotid space and right upper jugular region. After clinical evaluation, cardiac pacemaker was placed, but he still suffered from the syncope. Then, he received the chemotherapy with docetaxel and cisplatin. The last hypotension event occurred on day 10 of the chemotherapy. Six months after 3 cycles of chemotherapy, he remained in complete remission and resolution of syncope. We report a case in which syncope was associated with a recurrence of tonsilar cancer and successfully treated with chemotherapy.
Syncope is a difficult entity to assess. There are various causes of syncope, including carotid artery hypersensitivity and glossopharyngeal irritation. In the setting of head and neck cancer (HNC), syncope usually arises from mechanical compression of the carotid sinus and tumor-induced irritation of the glossopharyngeal nerve.1,2 Attempts to relieve syncope have included vasoconstrictive drugs, cardiac pacemaker placement, radiotherapy, and chemoradiothrapy.3 Pacemaker therapy might be effective if syncope is a cardioinhibitory reflex type with bradycardia. However, pacing unfortunately may not alleviate syncope which results from a pure vasodepressor syncope.4 Study on the role of chemotherapy in syncope of head and neck cancer has rarely been reported.5 We describe a patient who suffered from the recurrent syncope due to tumor compression of the carotid sinus, and initially failed with pacemaker but successfully treated with chemotherapy.
A 59-year-old man presented with recurrent syncopal episodes lasting for about 30 seconds and headache. Syncope was heralded by a sensation of lightheadedness.
The patient was hospitalized for further assessment. After second day of admission, he had recurrent syncopal episodes associated with seizure and hypotension. The blood pressure was 50/30 mmHg with 20 beat per minute. There was no clear precipitant for the syncopal attacks and atropine was given immediately. Consciousness and normal heart rate were retrieved.
Initial assessment included a computed tomography (CT) scan of the brain, electroencephalography (EEG) and neurologic exam which were unremarkable. However, 24h Holter monitoring showed long sinus pauses up to 3 seconds, correlating with syncope (Fig. 1). The syncope was presumed due to sick sinus syndrome. A dual chamber permanent pacemaker was placed and functioned normally. He was discharged, but was readmitted 1 week later with persisting syncope and left ear pain which occurred several times a week and occasionally associated with generalized rigidity. When syncope was developed, the pulse rate was normal but systolic blood pressure of 80 mmHg was observed. The patient recovered syncope spontaneously.
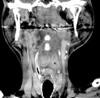
Consultation with the ear-nose-and-throat services was requested for ear pain, and the hypopharyngeal mass was found incidentally. Subsequently, CT scan of the neck was performed and showed ill-defined lesions in the left parapharyngeal, carotid spaces and right upper jugular region (Fig. 2). A biopsy of the hypopharyngeal mass revealed moderately differentiated squamous cell carcinoma.
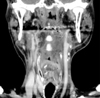
His past medical history included tonsilar cancer which had been diagnosed 4 years ago. The patient underwent wide excision of left tonsilar area and modified radical neck dissection (MRND). Subsequently he received adjuvant radiotherapy of 5,040 cGy dose. The syncope was thought to be associated with the cancer invasion in the carotid sinus. Because the patient had already received radiotherapy and operation, chemotherapy was considered for the treatment. Therefore, Docetaxel (25 mg/m2 on day 1, 8 and 15 in every 4 weeks) and Cisplatin 35 mg/m2 (on day 1, 8 and 15 in every 4 weeks) were administered. The last hypotensive event occurred on day 10 of chemotherapy and never recurred again. CT scan performed after 3 cycles of chemotherapy showed a complete resolution of tumor (Fig. 3). Six cycles of chemotherapy were initially planned for a period of 6 months. However, the patient experienced recurrent pneumonia and severe vomiting even after dose reduction and could not continue the chemotherapy. Six months after 3 cycles of chemotherapy, he remained in complete remission and resolution of syncope.
Syncope from HNC is uncommon, and there are several previous reports to describe it.6,7 Most reported cases of syncope are caused by carcinoma, but neurofibroma and lymphoma have also been reported.8,9 The exact mechanism of syncope from HNC is not well characterized, but it is similar to that of the carotid sinus syndrome (CSS).10 An abnormally strong carotid sinus reflex is believed to cause syncope from HNC. Weiss and Baker in their classic description of CSS classified the cardiovascular responses which leads to syncope to three forms: the cardioinhibitory type (asystole or marked bradycardia, with or without hypotension), the vasodepressor type (profound hypotension without bradycardia) and the rare cerebral type (without bradycardia or systemic hypotension).11,12 In cardioinhibitory syncope the efferent arc is mainly vagal, affecting the heart predominantly. In vasodepressor syncope, the efferent arc is thought to be mainly sympathetic, affecting mostly the peripheral vasculature. Pure vasodepressor syncope is thought to account for only 5-10% of cases of carotid sinus syncope, and the remainder is cardioinhibitory type.13 The significant bradycardia was obtained in 24 hours ECG (Holter) recording when our patient had syncope, thus indicating that our case was the cardioinhibitory type.
Syncope from HNC is different from CSS in several aspects. In the case of underlying neck malignancy, the attacks are usually spontaneous and carotid sinus massage does not characteristically provoke syncopal attacks.14 The syncope in HNC does occur not only when a tumor infiltrates the afferent nerve fibers of the glossopharyngeal nerve, but also the baroreceptor within the carotid sinus. Acute unilateral head or neck pain usually precedes the syncopal episode from which patients may be aware of an oncoming attack.15 Brief seizures have also been reported to accompany syncope both from hypersensitive carotid sinus and glossopharyngeal neuralgia.16 Seizures occur mainly during prolonged periods of asystole or with profound hypotension. In our case study, there was no pain in the pharyngeal region prior to syncopal attack, but he had brief seizure.
There is no known single effective treatment for patients. A variety of treatment might be considered to relieve syncope. Such attempts include vasoconstrictive drugs, cardiac pacemaker, radiotherapy and surgical resection of the glossopharyngeal nerve.4 Pacemaker therapy might relief syncope resulting from a cardioinhibitory reflex with bradycardia. However, pacing would not lessen syncope if pure vasodepressor syncope develops.3 In our case, the pacemaker had initially been placed, but the patient did not respond. Radiotherapy is effective in CSS in HNC. Radiotherapy of the carotid sinus might control syncope in up to 2/3 of patients with carotid sinus hypersensitivity.1 However, radiation has a drawback of delayed effect and concern about the hazard of radiation. In our case, the patient could not be treated with radiotherapy, because he was already given considerable doses of radiation in the neck region. Interruption of the glossopharyngeal nerve or carotid sinus by surgery has been shown to be effective in preventing syncope from carotid sinus hypersensitivity, even though it is often palliative.17
Treatment of syncope of HNC with chemotherapy alone has not been adequately documented.5 Chemotherapy showed moderate efficacy in HNC. The most active agents include cisplatin, paclitaxel, docetaxel, 5-FU and cetuximab. Especially, among newer agents, the taxanes seem to be promising. Single-agent therapy with semisynthetic taxane docetaxel demonstrated response rates from 27% to 42%.18 Weekly docetaxel and cisplatin regimen appeared to have a good activity and toxicity profile in HNC.19 Randomized trial has demonstrated significantly higher response rates for the combination regimen than single agents.20 In our case, chemotherapy was the only option for treatment. Therefore, Docetaxel and Cisplatin were given weekly to relieve symptoms. Our patient achieved complete remission of tumor and resolution of syncope with four cycles of chemotherapy. However, he could not receive more chemotherapy due to recurrent neutropenia and pneumonia. Ten months after diagnosis and treatment, the patient had no recurrence of syncope. Repeat CT scans and PET showed complete resolution of the lesion.
Figures and Tables
References
1. Macdonald DR, Strong E, Nielsen S, Posner JB. Syncope from head and neck cancer. J Neurooncol. 1983. 1:257–267.
2. Lin RH, Teng MM, Wang SJ, Yeh TP, Liao KK, Liu HC. Syncope as the presenting symptom of nasopharyngeal carcinoma. Clin Neurol Neurosurg. 1994. 96:152–155.
3. Chen-Scarabelli C, Kaza AR, Scarabelli T. Syncope due to nasopharyngeal carcinoma. Lancet Oncol. 2005. 6:347–349.

4. Bauer CA, Redleaf MI, Gartlan MG, Tsue TT, McColloch TM. Carotid sinus syncope in head and neck cancer. Laryngoscope. 1994. 104:497–503.

5. Choi YM, Mafee MF, Feldman LE. Successful treatment of syncope in head and neck cancer with induction chemotherapy. J Clin Oncol. 2006. 24:5332–5333.

6. Hong AM, Pressley L, Stevens GN. Carotid sinus syndrome secondary to head and neck malignancy: case report and literature review. Clin Oncol (R Coll Radiol). 2000. 12:409–412.

7. Hawkins J, Lewis HD, Emmot W, Vacek JL. Vasodepressive carotid sinus hypersensitivity with head and neck malignancy: treatment with propranolol. Am Heart J. 1991. 122:234–235.
8. Sutherland JA, Stobie P, Swarup V, Tierney SP, Lin AC, Burke MC. Hypersensitive carotid sinus syndrome due to neurofibromatosis-1 and manifested by repeated episodes of syncope. Pacing Clin Electrophysiol. 2004. 27:1571–1573.

9. Venkatraman V, Lee L, Nagarajan DV. Lymphoma and malignant vasovagal syndrome. Br J Haematol. 2005. 130:323.

10. Worth PF, Stevens JC, Lasri F, Brew S, Reilly MM, Mathias CJ, Rudge P. Syncope associated with pain as the presenting feature of neck malignancy: failure of cardiac pacemaker to prevent attacks in two cases. J Neurol Neurosurg Psychiatry. 2005. 76:1301–1303.

11. Weiss S, Baker JR. The carotid sinus reflex in health and disease: its role in the causation of fainting and convulsions. Medicine. 1933. 12:297–354.
12. McIntosh SJ, Lawson J, Bexton RS, Gold RG, Tynan MM, Kenny RA. A study comparing VVI and DDI pacing in elderly patients with carotid sinus syndrome. Heart. 1997. 77:553–557.

13. Walter PF, Crawley IS, Dorney ER. Carotid sinus hypersensitivity and syncope. Am J Cardiol. 1978. 42:396–403.

14. Johnston RT, Redding VJ. Glossopharyngeal neuralgia associated with cardiac syncope: long term treatment with permanent pacing and carbamazepine. Br Heart J. 1990. 64:403–405.

16. Thomas JE. Hyperactive carotid sinus reflex and carotid sinus syncope. Mayo Clin Proc. 1969. 44:127–139.
17. Dykman TR, Montgomery EB Jr, Gerstenberger PD, Zeiger HE, Clutter WE, Cryer PE. Glossopharyngeal neuralgia with syncope secondary to tumor. Treatment and pathophysiology. Am J Med. 1981. 71:165–170.
18. Dreyfuss AI, Clark JR, Norris CM, Rossi RM, Lucarini JW, Busse PM, et al. Docetaxel: an active drug for squamous cell carcinoma of the head and neck. J Clin Oncol. 1996. 14:1672–1678.

19. Guntinas-Lichius O, Appenrodt S, Veelken F, Krug B. Phase II study of weekly docetaxel and cisplatin in patients with advanced recurrent and metastatic head and neck cancer. Laryngoscope. 2006. 116:613–618.

20. Browman GP, Cronin L. Standard chemotherapy in squamous cell head and neck cancer: what we have learned from randomized trials. Semin Oncol. 1994. 21:311–319.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download