Abstract
Hydatid disease is a parasitic infestation caused by the larval form of Echinocococcus. In human, the most commonly affected organs are liver and lung. Most cysts remain clinically silent and are diagnosed incidentally or when complications occur. In Korea, hydatid disease is rare and surgically treated cases have been reported in the Korean literature. However, it is expected to confront this disease sooner or later, because of recent increase in traveling to the endemic area and industrial workers originating from those areas. With this trend, we experienced a case of hydatid cyst of the liver in a male patient from Uzbekistan. This patient was presented with anaphylactic shock combined with hydatid cyst. We successfully treated using ultrasound-guided transhepatic percutaneous drainage [termed puncture, aspiration, injection, and re-aspiration (PAIR)] of the hydatid cyst and concomitant albendazole instead of surgery. In this clinical case report, we describe all the course of the patient and recommend the PAIR as a first choice method for treatment of hepatic hydatid cyst.
Hydatid disease is still endemic in various regions of the world. Although it is the most frequent cause of liver cysts worldwide, it is rare in Korea and only a few cases have been reported.1,2 However, because of the recent increase of travel to endemic areas and industrial workers originating from those areas, it is expected that this disease will be encountered in other non-endemic countries. The rupture of a hydatid cyst commonly gives rise to an allergic response, which includes anaphylactic shock. Traditionally, surgery has been the only accepted mode of treatment, however, percutaneous treatment has recently been proposed as an alternative.3
Here, we describe a patient who presented with anaphylactic shock combined with hydatid cyst. This patient was successfully treated using ultrasound-guided transhepatic percutaneous drainage [termed puncture, aspiration, injection, and reaspiration (PAIR)] of the hydatid cyst and concomitant albendazole. This procedure was applied for the first time in Korea.
A 30-year-old man was admitted to our emergency room because of sudden loss of consciousness. He was an Uzbek industrial trainee. On physical examination, the patient appeared acutely ill and pale, and had a deep-red erythema, predominantly of the trunk and face. He had a temperature of 37.4℃, a respiratory rate of 28 breaths/min, and a pulse of 104 beats/min. His blood pressure was 60/30 mmHg. There were normal breathing sounds, and oxygen saturation was 100%.
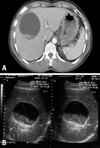
Laboratory tests (reference values in parentheses) on admission showed a level of C-reactive protein 6.73 mg/dL (< 0.5 mg/dL), a leukocyte count 15,100 cells/µL with 91.1% neutrophils (4,800-10,800 cells/µL), total protein 3.6 g/dL (6-8.3 g/dL), albumin 2.1 g/dL (3.5-5 g/dL), and no signs of liver damage and cardiac damage. The chest X-ray and abdomen were unremarkable with no focus of infection identified. Anaphylactic reaction was suspected. The patient was treated with a constant infusion of crystalloid and dopamine. The patient's condition improved rapidly. However, he had low-grade fever and vague pain in the right upper quadrant without muscle rigidity 3 days later. The abdominal ultrasound and CT demonstrated a unilocular cyst measuring 9×8 cm in the right liver lobe, which was suspected to be hydatid disease (Fig. 1). A clinical diagnosis of anaphylactic shock combined with hydatid cyst leakage was made. The patient was treated with albendazole. After 5 days, we performed ultrasound-guided aspiration of the contents of the cyst and recovered clear, colorless, gelatinous fluid. Hydatid sand containing a protoscolex of Echinococcus granulosus was observed on microscopic examination (Fig. 2).
On next day, we performed PAIR of the hydatid cyst without immediate complications. After -40 mL was aspirated, hypertonic saline and ethanol were administered as scolicidal agents through an indwelling catheter for 30 min. Two days later, scolicidal agents were instilled once more. Cyst aspirate was confirmed as dead scolex, and we removed the indwelling catheter. Five days later, a high fever developed. We considered the possibility of cyst infection and applied antibiotics, which led to clinical improvement of the patient. On day 33 after admission, the patient was discharged and received albendazol for 2 months. The patient returned to his normal level of activity. Follow-up ultrasonography of the abdomen, which was performed afterward, showed inactive status, including detachment of the membrane, echogenicity, pseudotumor pattern, and reduction in the size of the cyst. There has been no evidence of recurrent disease for 4 years.
Echinococcosis is a zoonosis caused by adult or larval stages of Echinococcus spp. Human infection occurs through the ingestion of parasite eggs from a carnivore host. Hydatid disease (larval infection) is characterized by the long-term growth of hydatid cysts in humans and intermediate hosts such as sheep, cattle, horses, and goats.4 Human beings become exposed to the eggs after close contact with an infected eggs of the infected dog or its contaminant environment.5 In Korea, there was a report of E. granulosus found in the lungs of cattle in Jeju-do,6 but it has been familiar to Koreans only as an imported tropical disease. Because of the increase in travel to endemic areas and industrial workers originating from those areas, we occasionally encounter this disease.
The liver is the most frequent site for the cystic lesions observed in hydatid disease, followed by the lung, brain, and other visceral organs.7 Once ingested, the ova are digested in the gastrointestinal tract of the host, and the larval form passes through the intestinal wall and travels to the liver via the portal circulation. If the larvae are not cleared by the liver, they are transported to the lungs and possibly the systemic circulation.
Most intact hydatid cysts produce no symptoms. The life span of hydatid cysts of E. granulosus can be as long as 53 years in humans.8 When symptoms do occur, they are usually caused by mass effects of the enlarging cyst in a confined space. Sometimes, cyst leakage or rupture by trauma may be associated with a severe allergic reaction. Antigenic hydatid fluid spillage sensitizes individuals and may cause an allergic response, varying from mild pruritus and urticaria to anaphylactic shock, convulsion, and coma. Anaphylaxis may happen spontaneously because high intracystic pressure causes leakage of cystic fluid into the circulation.9 In our patient, we could find no other causes of hypotension and comatose mental status, despite a through examination that included brain imaging, a culture study, echocardiogram, EKG, and other tests. Our patient came from Uzbekistan, an area that is endemic for hydatid cysts, and had a huge cyst, including a daughter cyst, in the liver. Therefore, we initially assumed that anaphylactic shock had developed spontaneously following spillage of hydatid cyst fluid and confirmed that the hydatid sand contained protoscoleces of E. granulosus. Although we didn't perform serology or immunoassay, hydatid disease can be confirmed by a combination of imaging techniques and serological techniques such as enzyme-linked immuno-sorbent assay.4
There are three therapeutic modalities for the treatment of hepatic hydatid cyst: chemotherapy, surgery, and percutaneous drainage. Traditionally, surgery has been the only accepted mode of treatment, and radical cystectomy and hepatectomy offer the best results. However, it has a high morbidity rate of 14-60%, an overall mortality of up to 7.8%, and leads to longer hospitalization.10 The results of treatment with only mebendazole or albendazole are controversial. Previously, percutaneous treatment of hepatic hydatid cyst has rarely been performed because of the fear of dissemination, peritoneal spillage, and anaphylactic shock, however, percutaneous drainage has been used successfully in recent years.3
The experts on hydatid disease recommend needle aspiration and concurrent chemotherapy instead of surgical removal.11,12 This technique, known as the PAIR procedure, satisfies all the goals of surgery for hydatid disease by sclerosing germinal membrane and separating laminated membrane with scolicides. The PAIR procedure is typically performed in three steps: puncture and needle aspiration of the cyst, instillation and indwelling of a scolicidal solution for 20-30 min, and cyst-re-aspiration and final irrigation. Patients who undergo PAIR typically receive oral albendazole or mebendazole for 7 days before and 28 days after drainage. Concomitant pre- and post-interventional chemotherapy offers the advantage of reducing the risk of disease recurrence and intraperitoneal seeding of infection, which may develop via cyst rupture and spillage that may occur spontaneously or during needle puncture. A metaanalysis indicated that PAIR plus chemotherapy is superior to surgical intervention in terms of disease recurrence, morbidity and mortality, and shorter length of stay.13
In our patient, even though anaphylactic shock associated with leakage occurred initially, PAIR therapy was performed successfully. Thus, PAIR therapy can be safely performed when spillage of hydatid fluid has already occurred. Another advantage of PAIR is that the activity of the hepatic hydatid cyst can be assessed by observing the viability of protoscolices in cytological specimens. In our case, hepatic hydatid cyst was diagnosed by visualizing protoscolices of E. granulosus in cyst aspirate, and successful outcome after PAIR was confirmed by the observation of dead scolices.
We performed follow-up examinations of our patient using periodic abdominal ultrasound for 4 years. We measured disease activity using the World Health Organization classification of cyst characteristics,14 which is widely accepted for the evaluation of the functional state of the parasite. Of types I to V, type IV (heterogeneous hyperechogenic degenerative contents, no daughter cysts) and type V (thick calcified wall, calcification partial to complete) are regarded as inactive. In our patient, initial ultrasonographic findings indicated type I features with a hyperechogenic cyst wall and hydatid sand. As treatment progressed, the final follow-up ultrasound examination revealed detachment of the germinal membrane and a heterogeneous pseudotumor pattern with ensuing calcification.
In conclusion, the differential diagnosis of anaphylactic reactions should include hydatid cyst, especially in patients who have cystic liver lesions and originate from countries where echinococcosis is endemic. In such cases, PAIR with medical treatment can safely be attempted. Ultrasound is recommended for follow-up examination of hydatid cyst activity.
Figures and Tables
Fig. 1
(A) Computed tomography of the abdomen with contrast medium showed a low-density cystic mass, measuring 9×8 cm, in the right hepatic lobe. (B) Ultrasonography of the liver showed a lesion with the characteristic appearance of a hydatid cyst with a hyperechogenic capsule and echogenic material.
References
1. Kang MJ, Lee SH, Kim SJ, Chei YH, Park JH, Park do H, et al. [A case of multiple intraperitoneal cysts from ruptured hepatic hydatid cysts]. Korean J Gastroenterol. 2007. 50:203–206.
2. Chai JY, Seo M, Suh KS, Lee SH. An imported case of hepatic unilocular hydatid disease. Korean J Parasitol. 1995. 33:125–130.
3. Filice C, Brunetti E, Bruno R, Crippa FG. Percutaneous drainage of echinococcal cysts (PAIR-puncture, aspiration, injection, reaspiration): results of a worldwide survey for assessment of its safety and efficacy. Gut. 2000. 47:156–157.
4. Zhang W, Li J, McManus DP. Concepts in immunology and diagnosis of hydatid disease. Clin Microbiol Rev. 2003. 16:18–36.

5. Seimenis A. Overview of the epidemiological situation on echinococcosis in the Mediterranean region. Acta Trop. 2003. 85:191–195.

6. Seo BS, Oh MY, Cho WY. An echinococcal cyst found in lung of cattle in Cheju-do. Korean J Parasitol. 1975. 13:85.
8. Spruance SL. Latent period of 53 years in a case of hydatid cyst disease. Arch Intern Med. 1974. 134:741–742.

9. Gelincik A, Ozşeker F, Büyüköztürk S, Colakoğlu B, Dal M, Alper A. Recurrent anaphylaxis due to non-ruptured hepatic hydatid cysts. Int Arch Allergy Immunol. 2007. 143:296–298.

11. Khuroo MS, Wani NA, Javid G, Khan BA, Yattoo GN, Shah AH, et al. Percutaneous drainage compared with surgery for hepatic hydatid cysts. N Engl J Med. 1997. 337:881–887.

12. Smego RA Jr, Sebango P. Treatment options for hepatic cystic echinococcosis. Int J Infect Dis. 2005. 9:69–76.

13. Smego RA Jr, Bhatti S, Khaliq AA, Beg MA. Percutaneous aspiration-injection-reaspiration drainage plus albendazole or mebendazole for hepatic cystic echinococcosis: a meta-analysis. Clin Infect Dis. 2003. 37:1073–1083.

14. WHO Informal Working Group. International classification of ultrasound images in cystic echinococcosis for application in clinical and field epidemiological settings. Acta Trop. 2003. 85:253–261.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download