Abstract
A 16-month-old boy was admitted because of cough that had lasted for 10 days. The patient showed severe hepatomegaly incidentally, and dual positivity of Immunoglobulin (Ig) M to Epstein-Barr virus (EBV) viral capsid antigen (VCA) and cytomegalovirus (CMV). On the basis of seroconversion to Epstein-Barr nuclear antigen (EBNA) Ig G positivity and reduced CMV Ig M titer with persistently negative CMV Ig G, a definite diagnosis of EBV-induced infectious mononucleosis was established 1 year 2 month later.
Infectious mononucleosis (IM) results from Epstein-Barr virus (EBV) and IM-like syndromes, mainly due to cytomegalovirus (CMV), Toxoplasma gondii or human immunodeficiency virus (HIV). EBV and CMV infection are common in humans. In immunocompetent persons, these infections are usually asymptomatic, however, can manifest as a severe infection in immunocompromised persons. The hallmarks of IM are fever, pharyngitis, lymphadenopathy, and a mononuclear cell count (lymphomonocytosis); ≥ 50% of the white blood cells (WBC) with atypical or reactive morphology of the lymphocytes in the peripheral blood.1,2 Additional features include splenomegaly or exanthema, and may contribute to the clinical diagnosis. We present a 16-month-old boy who had severe hepatomegaly which was detected incidentally during the admission period and was shown to have dual positive Immunoglobulin (Ig) M antibody to CMV and EBV. Initially, we thought that the patient had co-infection of CMV and EBV, however, after 1 year of follow-up, transient increase in CMV IgM was followed by persistent absence of CMV IgG and confirmation of EBV infection by serocon-version of IgG Epstein-Barr nuclear antigen (EBNA), suggesting that the CMV IgM test was false positive.
A 16-month-old Korean boy was admitted to our hospital in November, 2005, with a 10 days history of severe cough. He was diagnosed with an exudative tonsillitis and had been treated with oral antibiotics at the local clinic for 1 month. The cough became severe 10 days ago. The child was not so ill-looking; his weight was 10.0 kg (10-25 percentile), height 82.5 cm (75-90 percentile), temperature 36.9℃, pulse rate 120 per min, and respiration rate 40 per min. Physical examination revealed subcostal retraction, expiratory wheezing, whitish patches on pharynx, and diffuse abdominal distension with palpable liver about 10 cm below the righrt costal margin. Small multiple cervical lymph nodes were palpable in both sides. We had also experienced ampicillin-induced rash that showed erythematous, maculopapular rash on trunk and upper extremities. After removing it from the medication, rash had improved gradually.
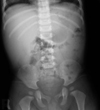
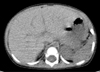
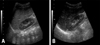
Complete blood cell count showed that hemoglobin was 12.3 g/dL, and leukocytes 11,300/mm3 (differential counts: 37/47/15). Peripheral blood smear showed neutrophilic leukocytosis with slight toxic granules, some atypical lymphocytes, and no blast cell. Respiratory syncytial virus antigen was negative and mycoplasma antibody titer showed 1 : 40 positive. Aspartate aminotransferase and alanine aminotransferase were 59 IU/L and 54 IU/L, respectively. His blood glucose, uric acid, and acid-base tests for the metabolic disorder as a cause of hepatomegaly were also normal. Screening test for viral hepatitis A, B, and C were all negative. Chest X-ray showed no active lesion. Abdominal X-ray and sonography showed severe hepatomegaly (liver margin reaching to the iliac crest) without splenomegaly (Fig. 1). However, no focal lesions in the liver, spleen or pancreas were detected by computed tomography (Fig. 2). Neck sonography showed bilateral cervical lymphadenitis. CMV and EBV viral test were done to evaluate the unknown origin of hepatomegaly as a cause of infection. CMV IgM and IgG were measured by means of enzyme immunoassay technique (BioMerieus, Lyon, France), and EBV viral capsid antigen (VCA) IgM, IgG, Epstein-Barr nuclear antigen (EBNA) IgM, IgG by means of enzyme immunoassay technique (Orgenics, Yavne, Israel). The positive values of EBV VCA IgM and IgG were defined more than 1.1 index and 1.1 U/mL, respectively. The positive value of EBV EBNA IgG was defined more than 1.1 index and IgM more than 12 index, respectively. The positive value of CMV IgM was defined more than 0.9 index and IgG more than 6 AU/mL, respectively. On day 3 of admission, IgM antibodies to CMV and EBV VCA were both positive. On day 4 of admission, cough and respiratory symptom improved and lung sound was clear. On day 6 of admission, serum aspartate aminotransferase and alanine aminotransferase 20 IU/L and 22 IU/L, respectively. On day 6 of admission, follow-up of complete blood cell count showed that hemoglobin was 11.7 g/dL, and leukocytes 20,800/mm3 (differential counts: 44/45/10). Severe hepatomegaly had lasted during 2 weeks and then its size decreased gradually. In the third week after admission, he was discharged, and since then, he had been followed up at our hospital with moderate hepatomegaly without symptom. One month later, VCA IgM showed seroconversion to VCA IgG and the decrease of CMV IgM antibody titer. Two month later, abdominal sonography showed mild hepatomegaly and seological test showed that VCA IgG and EBNA IgG were positive and both CMV IgM and IgG showed negativity. About 1 year 2 month later, the patient visited out patient clinic to evaluate his status and the abdominal sonography showed normal without hepatomegaly (Fig. 3) and serological tests showed that VCA IgG and EBNA IgG were positive and both CMV IgG and IgM persistently showed negativity (Table 1).
Hepatomegaly can be due to several mechanisms; it can be due to storage disease, inflammation, infiltration such as mass lesion, and so on. Primary infection of EBV may be seen at all ages, however, it is rarely apparent in children less than 4 years of age, since most EBV infections are asymptomatic. Our patient was 16 month-old and symptomatic EBV infection is rare in this age, nevertheless, the patient clinically seems to have an apparent EBV infection. Interestingly, in EBV infection, it is known that splenomegaly is common in 50% and hepatomegaly is seen only in 10%. CMV is also known to be causative agent of infantile hepatitis as well as EBV. CMV infection is one of the main causes of neonatal and infantile hepatitis. According to his age and clinical symptoms, we initially thought that the patient had co-infection of EBV and CMV. However, when he visited our clinic to check out his status after 1 year of disease onset, the follow-up results showed that VCA IgG and EBNA IgG were positive, while CMV IgG and IgM were persistently negative, which consistent with past EBV infection, not CMV infection.
An erythematous maculopapular eruption occurs in approximately 70% to 100% of patients with IM when antibiotics, specifically ampicillin, are administered during the acute stage of IM.3 Ampicillin produces a rash in approximately 3% to 22% of normal, healthy individuals without EBV infection.4 The increased incidence of the maculopapular rash that occurs in patients with IM who were treated with ampicillin suggests that there are specific factors associated with IM, resulting in this predisposition. In our patient, we also experienced ampicillin-induced rash that showed erythematous maculopapular rash on trunk and upper extremities. After eliminating it from the medication, the rash improved gradually.
Several mechanisms of simultaneous appearance of CMV IgM and EBV VCA IgM in infectious mononucleosis patients have been proposed such as co-infection of EBV and CMV,5-7 reactivation of EBV and CMV probably due to transient suppression of cellular immunity by CMV,8,9 and antigenic cross-reactivity among the herpes viruses including EBV and CMV.10-12 Cross-reaction to major antigenic epitopes between these elements and motifs has been reported to be a cause of false positive CMV IgM. However, it is essential to carefully interpret this combination EBV IgM, CMV IgM, EBNA IgG and CMV IgG for a diagnosis of a patient with infectious mononucleosis symptoms, since IgM CMV induced by CMV infection adversely cross-reacts with EBNA-1 protein. Inconsistent false positive rates of CMV IgM in infectious mononucleosis paitients range from 20.4% to 40.9%, and depend probably upon the specificity of kits, i.e. the antigenic CMV protein employed.7,11 Our patient was asymptomatic although severe hepatomegaly compared to other older adult case.12 To our regret, we were unable to test the CMV DNA in the blood during an acute stage because of noncooperation of parents. Nevertheless, our results indicate that the CMV IgM Axsym assay shows a lack of specificity in the acute stage of EBV infection, which had already been noted by Miendje Deyi, et al.11 Further studies will be needed in the future.
In conclusion, we experienced a patient with false positive CMV infection in the acute stage of EBV infection, and it is necessary to confirm the CMV infection by PCR, DNA technique, or seroconversion to CMV IgG.
Figures and Tables
References
1. Sumaya CV, Ench Y. Epstein-Barr virus infectious mononucleosis in children. I. Clinical and general laboratory findings. Pediatrics. 1985. 75:1003–1010.
2. Wakiguchi H, Hisakawa H, Kubota H, Kurashige T. Serodiagnosis of infectious mononucleosis in children. Acta Paediatr Jpn. 1998. 40:328–332.
3. Pullen H, Wright N, Murdoch JM. Hypersensitivity reactions to antibacterial drugs in infectious mononucleosis. Lancet. 1967. 2:1176–1178.
4. Ikediobi NI, Tyring SK. Cutaneous manifestations of Epstein-Barr virus infection. Dermatol Clin. 2002. 20:283–289.

5. Freigassner P, Ardjomand N, Radner H, El-Shabrawi Y. Coinfection of the retina by Epstein-Barr virus and cytomegalovirus in an AIDS patient. Am J Ophthalmol. 2002. 134:275–277.

6. Polz-Dacewicz M, Stec A, Koncewicz R. [CMC and EBV infections in children]. Przeql Epidemiol. 2002. 56:65–72.
7. Sánchez Echániz J, Mintegui Raso S, Benito Fernández J, Corral Carrancejo JM. [Mononucleosis syndromes with serology doubly positive to Epstein-Barr virus and cytomegalovirus]. An Esp Pediatr. 1996. 45:242–244.
8. Lang D, Vornhagen R, Rothe M, Hinderer W, Sonneborn HH, Plachter B. Cross-reactivity of Epstein-Barr virus-specific immunoglobulin M antibodies with cytomegalovirus antigens containing glycine homopolymers. Clin Diagn Lab Immunol. 2001. 8:747–756.

9. Bertram G, Dreiner N, Krueger GR, Ramon A, Ablashi DV, Salahuddin SZ, et al. Frequent double infection with Epstein-Barr virus and human herpesvirus-6 in patients with acute infectious mononucleosis. In Vivo. 1991. 5:271–279.
10. Rhodes G, Smith RS, Rubin RE, Vaughan J, Horwitz CA. Identical IgM antibodies recognizing a glycine-alanine epitope are induced during acute infection with Epstein-Barr virus and cytomegalovirus. J Clin Lab Anal. 1990. 4:456–464.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download