Abstract
Purpose
The aim of our study was to compare the efficacy of physical therapy alone and in combination with calcitonin in patients with neurogenic claudication (NC).
Materials and Methods
In this single blind, and randomized study, patients with lumbar spinal canal stenosis who were diagnosed by clinical findings and MRI and having NC were included. Patients were observed for 8 weeks and evaluated before and after treatment. Patients were randomized between the salmon calcitonin 200 U/day + physical therapy (n = 23) (Group 1) and paracetamol 1,500 mg/day + physical therapy (n = 22) (Group 2) treatment groups. Both groups received the same physical therapy (interferential current + hot pack + short wave diathermy) and exercise protocol. The association of various clinical and functional parameters was assessed statistically by using paired and unpaired t test, chi square test and McNemar's test. p < 0.05 indicated statistical significant.
Results
Mean age of the patients in Group 1 was 57.6 ± 11.2 and in Group 2 54.5 ± 10.6 years. Before treatment, there were no significant differences between groups with respect to age, body mass index, spinal axial diameter, Visual Analogue Scale (VAS), spinal mobility, functional status and walking distance (p > 0.05). After 8 weeks of treatment, both groups benefited significantly with respect to VAS, functional status and walking distance (p < 0.001). There was no statistically significant difference between groups (p > 0.05).
Lumbar spinal stenosis (LSS) is a clinical condition presenting with back and leg pain due to the compression of intraspinal vascular and neuronal structures by narrowed spinal canal. Pain, paresthesia, and cramping in one or both legs, described as neurogenic claudication, is present in 62% of patients, due to ischemia the nerve roots.1-3 Lumbar spinal anteroposterior diameter under 11 mm and a dural area below 100 mm2 in imaging procedures depict stenosis.4-6 However, as narrowing of the lumbar spinal canal is present in 20% of asymptomatic individuals, clinical findings are highly important in diagnosis and leg pain described as neurogenic claudication emerges as a valuable parameter in the observation of recovery.7
Conservative treatment is effective in LSS patients with mild or occasionally moderate pain.6 Daily life style adjustments, back training, exercise programs to stretch, strengthening the lumbar region, and general conditioning exercises both prescribed alone or together with physical therapy yield good clinical results.8-10
Calcitonin is a hormone secreted by the para-follicular cells of the thyroid, and used in the treatment of osteoporosis and Paget's disease. Due to its analgesic effect, it has also been used in LSS. Analgesic efficacy is considered to be through an increase of beta endorphin levels.11,12 Since the narrowing of the spinal canal is the result of soft tissue hypertrophy and edema, its success in treatment is due to anti-inflammatory and anti-edematous efficacy of calcitonin.13 Baker, et al.14 reported that, the deterioration of microcirculation in older patients causes neurogenic claudication in degenerative spinal stenosis, and calcitonin produces a decline in symptoms through its arterial dilator effect.
The aim of our study was to evaluate in a short term the effect of physical therapy alone and in combination with calcitonin on pain, physical examination results and the functional status of patients with neurogenic claudication, and diagnosis of lumbar spinal stenosis.
Patients referred to our outpatient clinic with lower back and leg pain, which described neurogenic claudication, and was diagnosed as LSS by physical examination and imaging studies were included in the study.
1) Pain in one or both legs after walking, diminishing of pain upon sitting or bending forward, and increase of pain with activities that increased extension of the spine such as descending stairs, 2) Restriction of spinal extension and pain in the low back/leg determined physical examination.
In order to exclude the presence of arterial claudication during physical examination, care was taken to ascertain palpable peripheral pulse and the absence of trophic disorders due to arterial insufficiency. Neurogenic claudication was also confirmed by the bicycle test. In the bicycle test, patients mounted on a static bicycle (Tunturi Static Bicycle) and instructed to push pedals for 5 minutes at a tolerable resistance.18 The absence of leg pain was evaluated in favor of neurogenic claudication.
After clinical assessment, patients with the narrowest level or levels with an axial diameter below 10 mm, measured by lumbar MRI, were included in the study by a definition of absolute stenosis, while those with an axial diameter of 10-12 mm were included by the definition of relative stenosis in the presence of clinical findings.19
Worsening of motor weakness, patients with bladder/gut dysfunction, previous spinal surgery, presence of inflammatory, infectious, or metastatic disease, neurological diseases affecting ambulation ability (such as stroke, Parkinsonism, peripheral entrapment neuropathy, etc), knee pain affecting ambulation ability, hip osteoarthritis, lower extremity peripheral arterial insufficiency, and current calcitonin use for osteoporosis.
In this single blind and randomized study, patients were consecutively randomized into 2 groups. Group 1 received 200 U/day intranasal calcitonin, while Group 2 received a maximum of 1,500 mg/day paracetamol. All patients took part in a physical therapy and exercise program. Physical therapy consisted of vacuum interference (20 min) + Hot Pack (20 min) + short wave diathermia (10 min) to the lumbar region, 5 days a week for 15 sessions. Exercises consisted of pelvic tilt, abdominal strengthening, hip flexion and hamstring stretching, and lumbar mobilization exercises.10 Exercises were taught and performed in the hospital by a physiotherapist during physical therapy session, and patients were thereafter recommended to repeat the same exercises twice a day at home.
Randomization (FY) and physical examinations (FS) were carried out by the same physiatrist, thus maintaining a single-blind design. No financial support was accepted for the study. The same nasal calcitonin product which could be obtained easily in the market was prescribed, and the other group was permitted to take paracetamol as analgesic. In order to evaluate the analgesic effect of calcitonin in a short term, a dosage of 200 U/day was prescribed, which is the dosage available in our country. All patients who would use calcitonin were informed about storage condition of the bottle (before opening, bottles were stored between 36-46 F in the fridge, and open bottles were stored at room temperature) and also application style. Patients were informed not to pump the calcitonin spray after 14 sprays, because each bottle contained 14 sprays.
Patients were evaluated at baseline and 8 weeks after treatment. Age, occupation, and Body mass index (BMI) of patients were recorded. Assessment parameters:
- Pain (at rest and with movement); patients were requested to evaluate the severity of pain on a 10 mm scale using the Visual Analogue Scale (VAS).
- Range of motion: measured by lumbar Schober (cm), finger-to-floor distance (cm), extension (degree-by lumbar goniometry).
- Functional status: evaluated by the Roland-Morris Scale.20
- Walking distance: expressed in meters.
The 24-item Roland-Morris Scale adapted to Turkish was used for functional assessment.20 In this scale, each item was answered by "yes" or "no", and "yes" responses took 1 and "no" responses 0 points, and the total score was calculated. A total score of 0 points meant "no disability", while a total score of 24 points meant "extremely severe disability".20
Walking distance was measured by a treadmill (Ferrox-Fulmine Treadmill) at 0° inclination, with the body at a vertical position, at a speed of 2 km/h.21 The test was terminated when patients suffered of leg pain, and walking distance was determined.
Statistical analyses were managed by the GraphPad Prisma V.3 program. Besides descriptive statistical methods (mean, standard deviation), independent t test in the comparison of paired groups, paired t test in the comparison of repeated measures in paired groups, the χ2-test in the comparison of qualitative data, and the McNemar's test in the comparison of repeated measures of qualitative data were used. Results were accepted significant at p < 0.05.
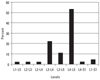
A total of 45 patients were included in the study. Group 1 consisted of 23, and Group 2 of 22 patients. The most commonly affected lumbar level was L4-L5, followed by narrowing at the L3-L4 level (Fig. 1).
Concomitant diseases were hypertension in 8, diabetes mellitus in 4, hypercholesterolemia in 4, ischemic heart disease in 2, and asthma in 1 patient.
In the group using calcitonin, only one patient had nasal irritation that did not necessitate cessation of therapy, and all patients were comfortable with the drug. In the group using paracetamol, patients used average 1.8 ± 0.8 tablets (each tablet of 500 mg). All patients who were enrolled in the study and completed the follow-up protocoland were included in the analysis.
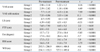
With respect to baseline values, there was no significant difference between groups in patients' age, gender, BMI values, axial diameter averages, VAS, lumbar ROM, functional status, and walking distance parameters (Table 1).
In the inter-group evaluation performed at week 8, lumbar Schober was similar in both groups, while the finger-to-floor distance significantly improved in the calcitonin group. All other parameters showed significant improvement in both groups (Table 2).
There was no significant difference between the groups with respect to improved parameters during the follow up period as well as their percent changes (Table 3).
The generally accepted practice for the treatment of LSS is the use of conservative therapy in mild and moderately symptomatic patients, and surgery in patients with severe symptoms.6,22,23 Another approach in patients in whom conservative treatment fails is to perform surgery.24-26 Naturally, conservative therapy is the treatment of choice in patients without motor disturbances and/or bladder or intestinal dysfunction.27 Trials examined the natural course of the disease actually confirm the use of conservative therapy as the first-line treatment. Johnsson, et al.,22 in the 49-month follow-up of 32 patients, determined improvement according to VAS, and found improvement in 15% of patients, no change in 70%, and worsening in 15%. Physical examination established improvement in 41% of patients, no change in 41%, and worsening in 18%. Sengupta and Herkowitz28 also states in his review that 15% of patients improved, 30% worsened within 2-3 years and needed surgery, while 45% were stable for a long time.
Conservative treatment options include physical therapy and exercise, epidural steroid injections, calcitonin and other analgesic/anti-inflammatory medications, and these are usually prescribed in combination.29 Studies on exercise treatment particularly emphasize the importance of flexion exercises, and recommend the addition of general conditioning exercises.8,10,30 Among trials using conservative treatment options, Onel, et al.31 combined physical therapy with superficial and deep heat with calcitonin, and implemented a one-month inpatient rehabilitation with an aggressive exercise program. Following the one-month observation period, 70% of patients achieved good outcomes. Simotas, et al.32 administered physical therapy to 96%, epidural steroid injections to 78%, acupuncture to 58%, and orthesis, bed rest, transcutanous electrical nerve stimulation (TENS) and manipulation to decreasing percentages of 40 patients with LSS, and observed decrease in especially pain parameters but no changes in functional assessments (walking distance, walking frequency) after a follow-up of average 33 months. The authors concluded that aggressive conservative treatment was a good option. In a study by Tadokoro, et al.,27 inpatient conservative therapy was administered to a group of patients with LSS over 70 years of age, and it was concluded after a follow-up of 57 weeks, that particularly those with radicular pain were good candidates for conservative treatment, and had a relatively good prognosis.
Our study also combined the conservative treatment options of physical therapy and exercise, and investigated the additive effect of short-term calcitonin as an analgesic. We administered low frequency currents for analgesia, Hot Pack for superficial heat, and short-wave diathermia for deep heat as physical therapy modalities. Exercises consisting particularly of lumbar flexion, mobilization, and stretching exercises were taught to patients during physical therapy sessions, and adviced to be repeated at home. Calcitonin was given at a dose of 200 U/day as a nasal spray, and evaluations were made at the end of 8 weeks.
The use of calcitonin in LSS was first published by Porter.33 In this study, 10 patients with neurogenic claudication symptoms were found to have improved with calcitonin, and calcitonin was considered to be affective by increasing the blood flow. A follwing study by Porter compared 100 U calcitonin with plasebo in 42 patients, and found no statistical significance although calcitonin increased walking distance.12 A study by Eskola, et al.13 with 15 patients followed for 3 months, also using 100 U calcitonin, and reported a decrease in pain and an increase in performance. Another double-blind, randomized placebo-controlled study by Eskola, et al.34 followed 39 patients for one year, and found that calcitonin was effective on the parameters like pain and walking distance, but less effective in patients with a walking distance below 200-300 meters. In another randomized, double-blind, controlled trial by Podichetty et al.35 with 36 patients with a VAS score over 6 which was observed for 6 weeks, no significant difference was found between 2 groups in terms of walking period, walking distance, or functional assessment by SF-36. Walking distance was limited to 130 m in this trial, and the authors suggested that an inadequate efficacy of calcitonin might be due to the severity of symptoms in this patient group. In our study, although the average pain score was 7.5 with VAS which was similar to other studies, walking distance was an average of 300 m which was longer than the others, i.e. symptom severity was also lower than others. In spite of these differences, calcitonin did not add any benefit to physical therapy and exercise program. In the present study, the Roland-Morris score used for functional assessment showed significant increase in all patients, but there was no significant difference between the 2 groups in terms of the percent change.
A comparison between conservative treatment and surgical therapy appears to be in favor of surgical therapy. In a long-term study with a large patient number, Atlas, et al.36 followed, 119 of 148 patients for 4 years. The operated group with more severe symptoms at baseline, compared with the non-operated group at the end of 4 years, showed significantly fewer symptoms, more treatment satisfaction, and better functional status. In the non-surgical group, improvement was modest and stable for 4 years. Amundsen, et al.25 followed 100 patients for 10 years, and reported that operated patients showed better outcomes and patients with severe symptoms especially benefited from surgery, and that conservative therapy should be administered to patients with moderate symptoms, as a delay in surgical treatment did not have negative impact on the outcome.
In conclusion, in our 45 patients with an average age of 55 years, neurogenic claudication, and moderate LSS symptoms in terms of walking distance, short-term physical therapy and exercise therapy for 3 months significantly improved pain, physical examination findings, and walking distance and functional parameters. The addition of 200 U/day calcitonin did not lead to a significant improvement in follow-up parameters. We, therefore, conclude that the addition of calcitonin as an analgesic in the short-term treatment of LSS along with physical therapy and exercise administration is not necessary.
Figures and Tables
Table 1
The Comparison of Patients' Age, Gender, BMI Values, Axial Diameter Averages and Pre-Treatment Follow-up Parameters

References
1. Amundsen T, Weber H, Nordal HJ, Magnaes B, Abdelnoor M, Lilleas F. Lumbar spinal stenosis: conservative or surgical management?: A prospective 10-year study. Spine (Phila Pa 1976). 2000. 25:1424–1435. discussion 1435-6.
2. Porter RW. Spinal stenosis and neurogenic claudication. Spine (Phila Pa 1976). 1996. 21:2046–2052.

3. Turner JA, Ersek M, Herron L, Deyo R. Surgery for lumbar spinal stenosis. Attempted meta-analysis of the literature. Spine (Phila Pa 1976). 1992. 17:1–8.
4. Shönström N, Lindahl S, Willén J, Hansson T. Dynamic changes in the dimensions of the lumbar spinal canal: an experimental study in vitro. J Orthop Res. 1989. 7:115–121.

5. Schonstrom NS, Bolender N, Spengler DM. The pathomorphology of spinal stenosis as seen on CT scans of the lumbar spine. Spine (Phila Pa 1976). 1985. 10:806–811.

6. Fritz JM, Delitto A, Welch WC, Erhard RE. Lumbar spinal stenosis: a review of current concepts in evaluation, management, and outcome measurements. Arch Phys Med Rehabil. 1998. 79:700–708.

7. Boden SD, Davis DO, Dina TS, Patronas NJ, Wiesel SW. Abnormal magnetic-resonance scans of the lumbar spine in asymptomatic subjects. A prospective investigation. J Bone Joint Surg Am. 1990. 72:403–408.

8. Fritz JM, Erhard RE, Vignovic M. A nonsurgical treatment approach for patients with lumbar spinal stenosis. Phys Ther. 1997. 77:962–973.

9. Simotas AC, Dorey FJ, Hansraj KK, Cammisa F Jr. Nonoperative treatment for lumbar spinal stenosis: clinical outcome results and 3-year survivorship analysis. Spine (Phila Pa 1976). 2000. 25:197–203.
10. Bodack MP, Monteiro M. Therapeutic exercise in the treatment of patients with lumbar spinal stenosis. Clin Orthop Relat Res. 2001. 384:144–152.

11. Porter RW, Hibbert C. Calcitonin treatment for neurogenic claudication. Spine (Phila Pa 1976). 1983. 8:589–592.

12. Porter RW, Miller CG. Neurogenic claudication and root claudication treated with calcitonin. A double-blind trial. Spine. 1988. 13:1061–1064.

13. Eskola A, Alaranta H, Pohjolainen T, Soini J, Tallroth K, Slätis P. Calcitonin treatment in lumbar spinal stenosis: clinical observations. Calcif Tissue Int. 1989. 45:372–374.

14. Baker AR, Collins TA, Porter RW, Kidd C. Laser Doppler study of porcine caudate equina blood flow. The effect of electrical stimulation of the rootlets during single and double site, low pressure compression of the cauda equina. Spine. 1995. 20:660–664.

15. Penning L, Wilmink JT. Posture-dependent bilateral compression of L4 or L5 nerve roots in facet hypertrophy. A dynamic CT-myelographic study. Spine (Phila Pa 1976). 1987. 12:488–500.

16. Takahashi K, Miyazaki T, Takino T, Matsui T, Tomita K. Epidural pressure measurements. Relationship between epidural pressure and posture in patients with lumbar spinal stenosis. Spine (Phila Pa 1976). 1995. 20:650–653.
17. Katz JN, Dalgas M, Stucki G, Lipson SJ. Diagnosis of lumbar spinal stenosis. Rheum Dis Clin North Am. 1994. 20:471–483.

18. Dyck P, Doyle JB Jr. "Bicycle test" of van Gelderen in diagnosis of intermittent cauda equina compression syndrome. Case report. J Neurosurg. 1977. 46:667–670.

19. Tuite GF, Stern JD, Doran SE, Papadopoulos SM, McGillicuddy JE, Oyedijo DI, et al. Outcome after laminectomy for lumbar spinal stenosis. Part I: clinical correlations. J Neurosurg. 1994. 81:699–706.
20. Küçükdeveci AA, Tennant A, Elhan AH, Niyazoglu H. Validation of the Turkish version of the Roland-Morris Disability Questionnaire for use in low back pain. Spine (Phila Pa 1976). 2001. 26:2738–2743.

21. Fritz JM, Erhard RE, Delitto A, Welch WC, Nowakowski PE. Preliminary results of the use of a two-stage treadmill test as a clinical diagnostic tool in the differential diagnosis of lumbar spinal stenosis. J Spinal Disord. 1997. 10:410–416.

22. Johnsson KE, Rosén I, Udén A. The natural course of lumbar spinal stenosis. Clin Orthop Relat Res. 1992. 279:82–86.

23. Johnsson KE, Udén A, Rosén I. The effect of decompression on the natural course of spinal stenosis. A comparison of surgically treated and untreated patients. Spine (Phila Pa 1976). 1991. 16:615–619.
24. Gunzburg R, Szpalski M. The conservative surgical treatment of lumbar spinal stenosis in the elderly. Eur Spine J. 2003. 12:Suppl 2. S176–S180.

25. Amundsen T, Weber H, Nordal HJ, Magnaes B, Abdelnoor M, Lilleâs F. Lumbar spinal stenosis: conservative or surgical management?: A prospective 10-year study. . Spine (Phila Pa 1976). 2000. 25:1424–1435.
26. Jolles BM, Porchet F, Theumann N. Surgical treatment of lumbar spinal stenosis. Five-year follow-up. J Bone Joint Surg Br. 2001. 83:949–953.
27. Tadokoro T, Miyamoto H, Sumi M, Shimornura T. The prognosis of conservative treatmants for lumbar spinal stenosis; analysis of patients over 70 years of age. Spine (Phila Pa 1976). 2005. 30:2458–2463.
28. Sengupta DK, Herkowitz HN. Lumbar spinal stenosis. Treatment strategies and indications for surgery. Orthop Clin North Am. 2003. 34:281–295.
29. Vo AN, Kamen LB, Shih VC, Bitar AA, Stitik TP, Kaplan RJ. Rehabilitation of orthopedic and rheumatologic disorders. 5. Lumbar spinal stenosis. Arch Phys Med Rehabil. 2005. 86:S69–S76.
30. Bridwell KH. Lumbar spinal stenosis. Diagnosis, management, and treatmant. Clin Geriatr Med. 1994. 10:677–701.
31. Onel D, Sari H, Dönmez C. Lumbar spinal stenosis: clinical/radiologic therapeutic evaluation in 145 patients. Conservative treatment or surgical intervention? Spine (Phila Pa 1976). 1993. 18:291–298.
32. Simotas AC, Dorey FJ, Hansraj KK, Cammisa F Jr. Nonoperative treatment for lumbar spinal stenosis. Clinical and outcome results and a 3-year survivorship analysis. Spine (Phila Pa 1976). 2000. 25:197–203.
33. Porter RW, Hibbert C. Calcitonin treatment for neurogenic claudication. Spine (Phila Pa 1976). 1983. 8:585–592.

34. Eskola A, Pohjolainen T, Alaranta H, Soini J, Tallroth K, Slätis P. Calcitonin treatment in lumbar spinal stenosis: a randomized, placebo-controlled, double-blind, cross-over study with one-year follow-up. Calcif Tissue Int. 1992. 50:400–403.

35. Podichetty VK, Segal AM, Lieber M, Mazanec DJ. Effectiveness of salmon calcitonin nasal spray in the treatment of lumbar canal stenosis: a double-blind, randomized, placebo-controlled, parallel group trial. Spine (Phila Pa 1976). 2004. 29:2343–2349.

36. Atlas SJ, Keller RB, Robson D, Deya RA, Singer DE. Surgical and nonsurgical management of lumbar spinal stenosis: four-year outcomes from the maine lumbar spine study. Spine (Phila Pa 1976). 2000. 25:556–562.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download