Abstract
Purpose
Methylphenidate (MPH) is an effective medication for the treatment of attention deficit hyperactivity disorder (ADHD). However, about 30% of patients do not respond to or are unable to tolerate MPH. Based on previous findings, we hypothesized that great variability in response time (RT) among Korean children with ADHD on a computerized continuous performance attention test would be related to poor MPH treatment response.
Materials and Methods
Children (ages 6-18 years) with ADHD were recruited for a prospective 12-week, open-labeled, multicenter study to examine optimal dosage of OROS methylphenidate. Of the 144 subjects selected, 28 dropped out due to adverse events, medication noncompliance, or follow-up loss, and an additional 26 subjects with comorbid disorders were excluded from statistical analyses. We defined 'responders' as subjects who received a score of less than 18 on the attention deficit hyperactivity disorder rating scale (ARS; Korean version, K-ARS) and a score of 1 or 2 on the Clinical Global Impression-Improvement scale (CGI-I). RT variability was assessed with the ADHD diagnostic system (ADS).
Results
Fifty-nine (67%) subjects responded to MPH treatment. The non-responders showed greater RT variability at baseline (Mann Whitney U = 577.0, p < 0.01). Baseline RT variability was a significant predictor of MPH response (Nagelkerke R2 = 0.136, p < 0.01). It predicted 94.9% of responder, 17.2% of non-responder and 69.3% of overall group.
Attention deficit hyperactivity disorder (ADHD) is a clinical disorder characterized by persistent hyperactivity, inattention, and impulsivity.1 Symptoms appear to be developmentally inappropriate and lead to functional impairments at home or in school.2 Children with ADHD are more likely to have comorbid learning difficulties and behavioral problems, and to have poor peer relationships and low self-esteem.3 About 30-50% of children with ADHD have significant behavior and psychiatric problems in adulthood.4-6 Persistence of ADHD symptoms into adolescence and adulthood is associated with antisocial behavior, substance use and abuse,7 fewer years of education, and lower rates of employment.8
Symptom control is strongly related to functional improvement.9-11 Methylphenidate (MPH) is recommended for the treatment of ADHD.12 Studies indicate that MPH is an effective remedy for both core symptoms (e.g., inattention, hyperactivity, and impulsivity) and aggressive behavior.13 MPH increases child compliance with parental commands and decreases hostile and negative responses, which facilitates the social interactions in young children.14-16 However, about 30% of children with ADHD do not tolerate or respond to stimulant medication.17,18 A study reported that the electroencephalogram (EEG) patterns of children who respond to MPH treatment are different from those who do not respond.19 However, little else is known regarding predictive factors for responsiveness to MPH. Further research into these predictive factors is warranted.
Studies have consistently shown that great response time (RT) variability is found in children with ADHD, making it a potential marker for differentiating children with ADHD from those without ADHD.20-26 Homozygosity for the DAT 10-repeat allele is correlated with poor response to MPH treatment among Korean children with ADHD.27 Furthermore, ADHD patients who have two copies of the 10-repeat allele at the dopamine transporter gene (DAT) show greater variability in RT on attention tests compared to those with fewer than two copies.28 These data suggest the possible relationship between greater RT and poor response to MPH treatment. We performed this study to examine whether great RT variability would be predictive of poor response to MPH treatment in children with ADHD.
Participants were recruited from seven sites in Korea for a prospective 12-week, open-labeled study to examine optimal dosage of OROS methylphenidate. Diagnoses were made with the Kiddie-Schedule for Affective Disorders and Schizophrenia-Present and Lifetime Version (K-SADS-PL).29 Exclusion criteria were as follows: use of MPH hydrochloride other than OROS-MPH within the past 24 hours; use of OROS-MPH within the past three months; use of psychotropic medication within the past four months (clonidine or other α-adrenaline agonist, tricyclic antidepressant, selective serotonin reuptake inhibitor, theophylline, coumarin, or anticonvulsant, antipsychotics, benzodiazepine, modafinil); history of hypersensitivity reaction to MPH hydrochloride or another component of OROS MPH; other medical problems, such as gastrointestinal disorders, glaucoma, cardiovascular disease, or hyperthyroidism; neurological illnesses, such as a seizure disorder; comorbid psychiatric disorders, such as pervasive development disorder, psychotic disorder, or Tourette syndrome; an intelligence quotient (IQ) less than 70 [as assessed by the Korean Wechsler Intelligence Scale for Children (K-WISC-III)30]; history of substance use or abuse; and possible pregnancy. After an initial assessment, 144 subjects (ages 6-18 years) with ADHD were included.
Baseline MPH dosages were either 18 mg or 27 mg depending on clinical judgment. Dosage was titrated for nine weeks, at which point it was maintained for the remainder of the 12-week treatment trial. Of the 144 subjects enrolled in the study, 28 dropped out due to adverse events (n = 8, 28.6%), medication noncompliance (n = 12, 42.9%), or follow-up loss (n = 6, 21.4%). Data from an additional 24 subjects with comorbid disorders, such as oppositional defiant disorder (n = 12), tic disorder (n = 9), depressive disorder (n = 3), and anxiety disorder (n = 6), were excluded. Since the ADHD diagnostics system was standardized for children of 6-15 years, we excluded 2 subjects who were older than 15. Thus, data from 88 participants were included in the final statistical analyses.
This study was approved by the Institutional Review Boards of all seven sites.
The attention deficit hyperactivity disorder rating scale (ARS; Korean version, K-ARS) is an 18-item measure used to assess inattention and hyperactivity. Items are rated on a 4-point scale (0 = never or rarely, 3 = very often). The K-ARS has good reliability and validity among Korean children.31 The IOWA Conners Parent Rating Scale (CPRS) is a 10-item measure administered to parents to assess inattention/overactivity (5 items) and oppositional/defiant behavior (5 items) in children.32 The CPRS has a 4-point rating scale (0 = never, 3 = very often) and it has good reliability and validity.32 The Clinical Global Impression Severity (CGI-S) and Clinical Global Impression Improvement (CGI-I) scales are clinical outcome measures. Both are clinician-administered and consist of 7-point scales (1 = much improved, 7 = much worse). Generally, the CGI-I is more sensitive to treatment effect.33 We defined 'responders' as subjects who received a score of less than 18 on the K-ARS and a score of 1 or 2 on the CGI-I, and ultimately were in remission state.34 Clinical assessment was done at baseline as well as weeks 1, 3, 6, 9, and 12. Body weights and vital signs were measured at every visit. ECG and laboratory measures were assessed at baseline and week 12.
The ADHD diagnostic system (ADS) is a computerized continuous performance test that consists of auditory and visual modalities.35 In each modality, the targets and non-targets are presented in the form of auditory or visual stimuli. The test can be used to assess children over five years of age. It consists of three sessions: the early, middle, and late phases. The ADS generates four broadband scores. The omission errors score indicates the number of times when the subject failed to respond to the target, with high scores reflecting inattention. The commission errors score indicates the number of times when the subject made an incorrect response to the non-target, with high scores reflecting impulsivity. The response time (RT) score measures the amount of time between presentation of the target stimulus and a correct response. RT is related to speed of information processing and motor response. The standard deviation of the RT reflects variability or consistency of attention.35 Scores are reported as T-scores and produced in a printable report. In our study, each subject performed the ADS at baseline and at the end of the study. We used scores derived from the visual modality of the ADS in light of research suggesting that the auditory modality is more difficult than the visual modality and is less sensitive in differentiating subjects with ADHD from healthy controls.35
To examine the effect of the RT variability on the MPH treatment response, we carried out analysis in two phases. At the first phase, we conducted T-tests, chi-square tests, Mann-Whitney U tests and univariate analysis of variance (ANOVA) to determine whether the responders differed significantly from the non-responders on the focal variables and extract the predictor variables. The RT variability was entered as an independent variable and commission error score was entered as a covariate for univariate ANOVA. Secondly, to examine the effect of the baseline RT variability on the MPH response, we conducted a binary logistic regression analysis, with baseline RT variability entered as the predictor variable, and "responder/non-responder" as the dependent variable. We ran a series of paired T-tests to compare the baseline and 12 week ADS scores. We then computed correlations to examine the relationship between the ADS scores and the behavior rating scale scores.
The mean age of the participants at baseline was 9.43 years (± 2.2). Slightly more than 87.5% (n = 77) of the children were boys and 11 (12.5%) were girls. The average IQ was 109.7 (± 16.1). The mean K-ARS and CPRS scores were 28.2 (± 8.5) and 11.7 (± 5.3), respectively.
Characteristics of the responders (n = 59) and non-responders (n = 29) are shown in Table 1. There were no significant differences between the responders and non-responders for age or gender. Nor were there any significant differences between the responders and non-responders for baseline IQ, K-ARS, and CPRS scores.
At week 12, the mean dosage of MPH across subjects was 0.99 mg/kg (± 0.29). There was no statistically significant difference in week 12 MPH dosage between the responders and non-responders. At week 12, the responders scored significantly lower on the K-ARS and CPRS compared to the non-responders (Table 1).
The ADS scores are summarized in Table 2. At baseline, the result of univariate ANOVA showed that the non-responders had significantly greater RT variability compared to the responders (F = 5.330, p = 0.028). However, there was no significant difference in commission error between responders and non-responders. The non-responder RT variability at week 12 was 78.0 (± 39.5), which is out of normal range and has clinical significance.35
There was a significant decrease in commission errors and RT variability from baseline to week 12 among the responders (Fig. 1). The means of all four scores fell within the normal range. Similarly, there was a significant decrease in commission errors and RT variability from baseline to week 12 among the non-responders (Fig. 2). However, among the non-responders, RT variability was greater than 70 even after 12 weeks of treatment.
The ADS scores were not correlated with the K-ARS and CPRS scores at baseline or at week 12.
RT variability at baseline was a significant predictor of treatment response at week 12 (Nagelkerke R2 = 0.136, p < 0.01). It predicted 94.9% of responder, 17.2% of non-responder and 69.3% of overall group.
In the present study, the non-responders showed greater RT variability at baseline. Great RT variability may reflect dysfunctional prefrontal activity and inefficient top-down control of attention.36 In a longitudinal study to evaluate the neuropsychological functions of children with ADHD, Halperin, et al.37 reported that a deficiency in response variability remained at the 10-year follow-up regardless of remission status. Great RT variability seems to indicate high risk in terms of the persistence of deficit in ADHD. Our results together, with those of earlier studies20,37 suggest that patients with great RT variability may have more severe neuronal dysfunction and high RT may indicate poor outcome.
We found no significant correlations between RT variability and the K-ARS and CPRS scores, which is in line with previous study, indicating that scores on continuous performance tests have low correlations with ADHD clinical symptom severity.38 In spite of the significant difference in baseline RT variability between the responders and non-responders, there was no significant difference in baseline clinical symptom severity between the responders and non-responders. This suggests that MPH treatment responsiveness is independent of baseline clinical symptom severity. Furthermore, regardless of the responsiveness to treatment, we found significant decreases in commission and RT variability after treatment. This also supports previous finding to show low correlation between clinical symptoms and CPT test results.
It is generally accepted that MPH can decrease the errors of omission.39 In the present study, there was a tendency to decrease in the errors of omission after treatment, however it did not reach statistical significance. Small sample size would be a possible explanation.
To our best knowledge, we are the first to present findings to indicate that great RT variability may predict poor response to MPH treatment. Although Coghill, et al.40 examined whether baseline neuropsychological functioning predicted clinical response to MPH, they did not include a measure of RT, therefore, their results could not be compared to ours.
This study has several limitations that should be noted. First, a placebo control group was not included in the study design. Second, we did not perform a time series analysis. Since ADS program only preserves summary data and converts T scores after completion of the task, we were unable to collect response time from each response. Studies using time series analyses of RT data suggest that RT variability can be broken down into heterogeneous components.21,25 In a time series analysis of data gathered from a Sustained Attention to Response Task (SART), Johnson, et al.41 reported that RT variability has 'fast' and 'slow' components, and that the fast component of RT variability reflects moment-to-moment variability and is predictive of response to MPH treatment, whereas the slow component of RT variability reflects a gradual deterioration in RT variability and is not predictive of response to MPH treatment. They also found no significant difference between the subjects with ADHD and the healthy controls in slow variability RT, suggesting that this component of RT may not tap into the deficits associated with ADHD. We were unable to compare our findings with those of Johnson, et al., as we used a different measure of RT variability, but future research to determine the predictive powers of the fast and slow components of RT variability is needed using a time series analysis. Third, the worst responders might have been dropped out due to lack of compliance. The effect was still found, nevertheless, the effect seen in this study might be underestimated.
In spite of these limitations, the present study has implications for clinical work. Our finding to suggest that RT variability may be predictive of MPH treatment response provides clinicians with a possible clinical indicator to be used in treatment planning with MPH.
Figures and Tables
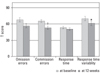 | Fig. 1ADHD diagnostic system scores of the responders. Baseline and week 12 scores were compared by paired T-test analyses. ADHD, attention deficit hyperactivity disorder. *p < 0.05, †p < 0.01. |
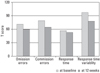 | Fig. 2ADHD diagnostic system scores of the non-responders. Comparison of baseline and week 12 scores were made by paired T-test analyses. ADHD, attention deficit hyperactivity disorder. *p < 0.05. |
Table 1
Characteristics of the Responders and Non-Responders
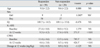
IQ, Intelligence quotient, as assessed by the Korean Wechsler Intelligence Scale for Children (K-WISC-III); K-ARS, Korean version of the ADHD Rating Scale; CPRS, IOWA Conners Parent Rating Scale.
Numbers are means (± standard deviations), except for sex, where numbers represent frequencies.
*χ2.
†Fisher's exact test.
‡Mann-Whitney U.
References
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 2000. 4th ed. Washington DC: American Psychiatric Association.
2. Barkley RA. Barkley RA, editor. Associated Cognitive, Developmental, and Health Problems. Attention-Deficit Hyperactivity Disorder: a Handbook for Diagnosis and Treatment. 2006. 3rd ed. New York: The Guilford Press;123–183.
3. Barkley RA. Comorbid Disorders, Social and Family Adjustment, and Subtyping. Attention-Deficit Hyperactivity Disorder: a Handbook for Diagnosis and Treatment. 2006. 3rd ed. New York: The Guilford Press;184–218.
4. Mannuzza S, Klein RG, Bessler A, Malloy P, LaPadula M. Adult outcome of hyperactive boys. Educational achievement, occupational rank, and psychiatric status. Arch Gen Psychiatry. 1993. 50:565–576.
5. Loeber R, Dishion T. Early predictors of male delinquency: a review. Psychol Bull. 1983. 94:68–99.
6. Gittelman R, Mannuzza S, Shenker R, Bonagura N. Hyperactive boys almost grown up. I. Psychiatric status. Arch Gen Psychiatry. 1985. 42:937–947.
7. Barkley RA, Fischer M, Smallish L, Fletcher K. Young adult follow-up of hyperactive children: antisocial activities and drug use. J Child Psychol Psychiatry. 2004. 45:195–211.
8. Morrison J. Adult psychiatric disorders in parents of hyperactive children. Am J Psychiatry. 1980. 137:825–827.
9. Swanson JM, Wigal SB, Wigal T, Sonuga-Barke E, Greenhill LL, Biederman J, et al. A comparison of once-daily extended-release methylphenidate formulations in children with attention-deficit/hyperactivity disorder in the laboratory school (the Comacs Study). Pediatrics. 2004. 113:e206–e216.

10. Biederman J. Pharmacotherapy for attention-deficit/hyperactivity disorder (ADHD) decreases the risk for substance abuse: findings from a longitudinal follow-up of youths with and without ADHD. J Clin Psychiatry. 2003. 64:Suppl 11. 3–8.
11. Cox DJ, Merkel RL, Penberthy JK, Kovatchev B, Hankin CS. Impact of methylphenidate delivery profiles on driving performance of adolescents with attention-deficit/hyperactivity disorder: a pilot study. J Am Acad Child Adolesc Psychiatry. 2004. 43:269–275.

12. Greenhill LL, Pliszka S, Dulcan MK, Bernet W, Arnold V, Beitchman J, et al. Practice parameter for the use of stimulant medications in the treatment of children, adolescents, and adults. J Am Acad Child Adolesc Psychiatry. 2002. 41:26S–49S.

13. Connor DF. Preschool attention deficit hyperactivity disorder: a review of prevalence, diagnosis, neurobiology, and stimulant treatment. J Dev Behav Pediatr. 2002. 23:S1–S9.

14. Barkley RA. The use of psychopharmacology to study reciprocal influences in parent-child interaction. J Abnorm Child Psychol. 1981. 9:303–310.
15. Barkley RA. The effects of methylphenidate on the interactions of preschool ADHD children with their mothers. J Am Acad Child Adolesc Psychiatry. 1988. 27:336–341.
16. Barkley RA. Hyperactive girls and boys: stimulant drug effects on mother-child interactions. J Child Psychol Psychiatry. 1989. 30:379–390.
17. Wilens TE, Biederman J. The stimulants. Psychiatr Clin North Am. 1992. 15:191–222.
18. Newcorn JH, Kratochvil CJ, Allen AJ, Casat CD, Ruff DD, Moore RJ, et al. Atomoxetine and osmotically released methylphenidate for the treatment of attention deficit hyperactivity disorder: acute comparison and differential response. Am J Psychiatry. 2008. 165:721–730.

19. Clarke AR, Barry RJ, McCarthy R, Selikowitz M, Croft RJ. EEG differences between good and poor responders to methylphenidate in boys with the inattentive type of attention-deficit/ hyperactivity disorder. Clin Neurophysiol. 2002. 113:1191–1198.

20. Castellanos FX, Sonuga-Barke EJ, Milham MP, Tannock R. Characterizing cognition in ADHD: beyond executive dysfunction. Trends Cogn Sci. 2006. 10:117–123.

21. Castellanos FX, Sonuga-Barke EJ, Scheres A, Di Martino A, Hyde C, Walters JR. Varieties of attention-deficit/hyperactivity disorder-related intra-individual variability. Biol Psychiatry. 2005. 57:1416–1423.

22. de Zeeuw P, Aarnoudse-Moens C, Bijlhout J, König C, Post Uiterweer A, Papanikolau A, et al. Inhibitory performance, response speed, intraindividual variability, and response accuracy in ADHD. J Am Acad Child Adolesc Psychiatry. 2008. 47:808–816.

23. Doyle AE. Executive functions in attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2006. 67:Suppl 8. 21–26.
24. Klein C, Wendling K, Huettner P, Ruder H, Peper M. Intrasubject variability in attention-deficit hyperactivity disorder. Biol Psychiatry. 2006. 60:1088–1097.

25. Johnson KA, Kelly SP, Bellgrove MA, Barry E, Cox M, Gill M, et al. Response variability in attention deficit hyperactivity disorder: evidence for neuropsychological heterogeneity. Neuropsychologia. 2007. 45:630–638.

26. Hurks PP, Adam JJ, Hendriksen JG, Vles JS, Feron FJ, Kalff AC, et al. Controlled visuomotor preparation deficits in attention-deficit/hyperactivity disorder. Neuropsychology. 2005. 19:66–76.

27. Cheon KA, Ryu YH, Kim JW, Cho DY. The homozygosity for 10-repeat allele at dopamine transporter gene and dopamine transporter density in Korean children with attention deficit hyperactivity disorder: relating to treatment response to methylphenidate. Eur Neuropsychopharmacol. 2005. 15:95–101.

28. Bellgrove MA, Hawi Z, Kirley A, Gill M, Robertson IH. Dissecting the attention deficit hyperactivity disorder (ADHD) phenotype: sustained attention, response variability and spatial attentional asymmetries in relation to dopamine transporter (DAT1) genotype. Neuropsychologia. 2005. 43:1847–1857.

29. Kim YS, Cheon KA, Kim BN, Chang SA, Yoo HJ, Kim JW, et al. The reliability and validity of Kiddie-Schedule for Affective Disorders and Schizophrenia-Present and Lifetime Version-Korean version (K-SADS-PL-K). Yonsei Med J. 2004. 45:81–89.

30. Kwak K, Park H, Kim C. The manual for the Korean WISC-III. 2002. Seoul: The Special Education.
31. So YK, Noh JS, Kim YS, Ko SG, Koh YJ. The reliability and validity of Korean parent and teacher ADHD rating scale. J Korean Neuropsychiatr Assoc. 2002. 41:283–289.
32. Shin MS, Ryu ME, Kim BN, Hwang JW, Cho SC. Development of the Korean version of the IOWA Conners Rating Scale. J Korean Neuropsychiatr Assoc. 2005. 44:82–88.
33. Arnold LE, Aman MG. Martin A, Scahill L, Charney DS, Leckman KF, editors. Clinical instruments and scales in pediatric psychopharmacology. Pediatric psychopharmacology. 2003. New York: Oxford University Press;412.
34. Michelson D, Allen AJ, Busner J, Casat C, Dunn D, Kratochvil C, et al. Once-daily atomoxetine treatment for children and adolescents with attention deficit hyperactivity disorder: a randomized, placebo-controlled study. Am J Psychiatry. 2002. 159:1896–1901.

35. Shin M, Cho S, Chun S, Hong K. A study of the development and standardization of ADHD Diagnostic system. Korean J Child & Adol Psychiatr. 2000. 11:91–99.
36. Bellgrove MA, Hester R, Garavan H. The functional neuroanatomical correlates of response variability: evidence from a response inhibition task. Neuropsychologia. 2004. 42:1910–1916.

37. Halperin JM, Trampush JW, Miller CJ, Marks DJ, Newcorn JH. Neuropsychological outcome in adolescents/young adults with childhood ADHD: profiles of persisters, remitters and controls. J Child Psychol Psychiatry. 2008. 49:958–966.

38. Naglieri JA, Goldstein S, Delauder BY, Schwebach A. Relationships between the WISC-III and the Cognitive Assessment System with Conners' rating scales and continuous performance tests. Arch Clin Neuropsychol. 2005. 20:385–401.

39. Losier BJ, McGrath PJ, Klein RM. Error patterns on the continuous performance test in non-medicated and medicated samples of children with and without ADHD: a meta-analytic review. J Child Psychol Psychiatry. 1996. 37:971–987.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download