Abstract
Hepatocellular carcinoma (HCC) is one of the most critical global health issues. With frequent association of viral liver disease, HCC is highly complex, harboring both cancer and chronic liver disease. The tumor stage and underlying liver function are both major determinants of the treatment selection as well as prognosis in HCC patients, thus allowing no more than a 20% chance for potentially curative therapies. Radiotherapy technology has been evolved remarkably during the past decade, and radiation can be precisely delivered, thereby permitting higher doses to the tumour and reduced doses to surrounding normal tissues. There has been increasing interest in the merits of radiotherapy in HCC over the past few years, as indicated by a Pub Med search. Radiotherapy has been used as the definitive therapy with curative intent in early stage tumours. It has been used also in combination with TACE for intermediate stage tumours. In locally advanced tumours, radiotherapy has been combined with systemic agents. Despite its efficacy, radiotherapy has not yet been incorporated into the standard management guidelines of HCC. The lack of high evidence level data, especially randomized controlled trials, has posed an obstacle in including radiotherapy into the routine treatment schema of HCC. Therefore, well-designed prospective studies are strongly recommended using developing technology for radiotherapy alone or combination therapies. Also, many issues such as the optimal dose-fractionation, intra- or extrahepatic metastasis after radiotherapy, and radiation-induced hepatic dysfunction remain to be solved. In this review, current status of radiotherapy for HCC will be discussed with regard to technical consideration and combination strategy. The limitation and future perspectives will also be discussed.
Hepatocellular carcinoma (HCC) is one of the critical global health issues. As indicated in cancer statistics, the disease is the third most common cause of cancer-related deaths worldwide.1 HCC is particularly prevalent in Asia, where hepatitis B virus (HBV) is endemic.2,3 Association of HCC with viral liver disease, which is a unique entity, places this cancer in a complex one to be properly managed.
In general, the tumor stage and underlying liver function are both major determinants of the treatment selection as well as prognosis in HCC patients.4 Potentially curative therapies are offered to only limited patients, which are estimated to be around 20%. Liver resection and liver transplantation are curative treatments. However, surgical resection accompanies high recurrence rate, and transplantation cannot be universally applicable. Radiofrequency ablation therapy and percutaneous ethanol injection therapy are also used as curative treatments for HCC.5 Because HCC is frequently diagnosed at the intermediate or advanced stages, transarterial chemoembolization (TACE), the most popular nonsurgical alternative, is often used as a palliative treatment,6,7 however, effective only in limited occasion. HCC is also resistant to current chemotherapeutic drugs.
During the past decade, there has been a major evolution regarding the management of HCC; emergence of the clinically proven drug for its efficacy and active use of radiotherapy using either external photon or particle beam. Sorafenib, a new targeting agent, is the first drug that showed survival benefit in randomized clinical trial.8,9 Despite of the significance shown in the trial, however, the substantial gain in survival remains so modest that the clinical usefulness is questioned in view of the cost-benefit aspect. Its benefit needs to be further tested in various therapeutic strategies.
Radiotherapy has not frequently been used for treatment of HCC. This limitation results from the early reports that radiation tolerance of liver was far less than the therapeutic radiation dose,10,11 hence, low level of therapeutic ratio. However, recent technological developments have enabled more successful treatment of HCC by delivering a substantial dose of radiation to the tumor and avoiding radiosensitive healthy normal organs in the vicinity. In Korea, radiotherapy of HCC has been initiated in a single institute.12-14 But now, the improved efficacy of radiotherapy has been more widely understood and resulted in increasing number of institutions adopting local radiotherapy for advanced HCC,15-18 as evidenced by soaring number of the published reports (Fig. 1).
However, it should be noted that HCC is a complex disease with cancer and chronic liver disease. It also has a great potential of intrahepatic and extrahepatic metastasis.19,20 Therefore, achieving the therapeutic success in HCC seems quite possible through a multimodal team approach, which is a basic condition for good performance of radiotherapy for HCC. Besides, there are many questions remaining unanswered. In this review, current status of radiotherapy for HCC will be discussed with regard to technical consideration and combination strategy. The limitation and future perspectives will also be discussed.
The basic strategy of radiotherapy is based on consideration of tumor control and normal tissue toxicity, which reflects radiosensitivity of the both elements. In radiotherapy of HCC, radiosensitivity of tumor and normal tissues haven't been in favor of therapeutic success. Until recently, radiosensitivity of HCC has been misunderstood as resistant. This notion came from poor clinical outcome of the early trials, which used insufficient radiation dose for fear of radiation-induced liver toxicity.21-23 Through the accumulated experiences using substantial doses, radiosensitivity of HCC has been revalued as sensitive.17,18,24 Some exemplary patients' cases are illustrated in Figs. 2 and 3.
Not many investigations have been performed on radiobiology of HCC. Recently, Tai, et al.25 has developed a radiobiologic model for primary liver tumors. Through analyzing 3 published clinical series of primary liver cancer treated with different dose fractionation, the radiobiologic parameters were extracted; and tumor doubling time (Td) were calculated as 15.0 ± 2.0 Gy, 0.01 ± 0.001, and 128 ± 12 day, respectively. This model has several weakness since it is based not on the individual clinical data, but on the median values of treatment parameters. Besides, it is based not on the tumor response, but on the survival. The study population also involves primary liver cancers other than HCC. However, a trial application showed well fit to a selective clinical sample. Through further modification, HCC-specific radiobiologic model is expected to be developed in the near future.
For normal tissue effects on radiation, the critical volume model has been proposed.26,27 Based on the assumption that normal tissue complication probability is determined by the fraction of surviving functional subunits, an organ is considered as the sum of multiple functional subunits which are arranged either serially or in parallel. Since liver belongs to parallel organ group, damage to a portion of liver may not impair the entire liver function. Because the remaining portion can maintain the organ function independently, overt complication occurs only when a critical volume of the organ is damaged. This is a key concept in radiotherapy of HCC; as long as the volume irradiated is limited, radiation dose can be escalated to the tumoricidal level without deteriorating liver function. Furthermore, liver is a unique organ that has a proficient regenerative potential. In fact, radiation dose could be escalated to higher than 70 Gy in a limited volume of the liver.28
In clinical application, situation looks more complex with several intercepting factors; presence of concurrent chronic liver disease in majority of patients and frequent use of combination treatment. Concurrent chronic liver diseases, which are more frequent in Asian patients, might deteriorate hepatic functional reserve. Combination treatment might also alter the hepatic functional reserve. In an animal experiment using rats with liver cirrhosis, concurrent treatment of partial liver radiation and 5-Fu chemotherapy significantly increased lethality.29 Therefore, radiation dose prescription needs to be tailored according to clinical situation, assuming that the hepatic tolerance to radiation would be modified.
Since the liver is in close proximity with many abdominal organs, toxicity of surrounding gastrointestinal organs should be taken into consideration. Normal tissue tolerance doses that have been defined as TD5/5 (tolerance dose representing the 5% complication rate 5 years after irradiation) and TD50/5 (tolerance dose representing the 50% complication rate 5 years after irradiation) remain as valuable guides.30,31 However, presence of other predisposing factors needs to be in consideration, including other medical illness, past history of cancer treatment as well as content of the present treatment such as concurrent chemotherapy. In general, stomach and small bowel should be limited to 45 Gy and colon to 50 to 54 Gy. Since these organs belong to serial type, attention should be paid not to generate focal overdose area beyond the tolerance level.
Technological advances in radiotherapy involve development in imaging technology as well as computer technology, and these advances have influenced the whole process of radiotherapy, ranging from treatment planning, and dose delivery to response assessment.
For planning, the bottom line will be CT-based conformal planning. Through this process, beam optimization can be possible for conformal coverage of the tumor as well as avoiding critical normal tissues. Computer calculation of dose-volume statistics provides information that can be used for dose prescription. Depending on the volume of normal liver to be saved, the dose to the gross tumor volume can be determined. Several published guideline suggests to deliver differential radiation doses by volume of normal liver to be irradiated (Table 1). Briefly, 3 types are popularly used in the published reports; volume parameter (V50%, 50% of the isocenter dose).14,32,33 normal tissue complication probability (NTCP) at 5% risk of radiation induced liver disease,34-36 and mean dose to normal liver.34,37 Consensus hasn't been reached yet regarding to which level radiation dose can be safely delivered. Therefore, this is an another area of future study.
Since the radiotherapy planning is a precise performance, beam delivery should also be closely monitored for presence of uncertainty in actual beam delivery. Considering that liver is a moving organ and moving distance also varies according to the anatomical position in liver segment, monitoring of uncertainties is particularly important in treating HCC. Generally, 2 types of uncertainties are identified; intra- and inter-fractional.38 The variability in liver position has been observed in non-breathhold stereotactic body radiotherapy, showing interfraction variability as an important source of geometric uncertainty with rather minimal extent in intrafraction change in liver motion.39 To mitigate unwanted problems arising from uncertainties, image guided radiotherapy (IGRT) technique is essential.
Clinically, several options of IGRT are in use.39-41 The simplest way is to cephalocaudally extend the physical target volume margin. This method needs fluoroscopic assessment of the organ motion for its excursion distance. Abdominal pressure or breath hold technique with active breath control can also be possible. Since the magnitude of breath control varies, these techniques basically require image guided monitoring. Another technique involves treatment of the entire moving path of the liver during free breathing using 4-dimensional CT scan imaging. In this technique, the timing of CT images and the position of external fiducial markers are synchronized. More advanced techniques involve gated radiotherapy and tumor tracking. In gated radiotherapy, external surrogates are used for reference point in monitoring tumor position. Finally, the most accurate method seems to be real-time tracking of tumor motion.42 In this technique, radio-opaque fiducial markers are implanted in or near the tumor. These markers are used to create a coordination system for image guidance involving real-time tracking of tumor motion. Through dynamic track-and treat options, gating of the treatment beam can be performed.
Although sophisticated technologies are developed, problems still exist; high cost in hardware and labor-intensive process involving treatment planning, beam delivery, and quality control. For better distribution and easier application in daily practice, the technology needs to be further simplified and cost-friendly.
Radiotherapy is classified to internal and external radiotherapy. Internal radiotherapy is performed by delivering radioisotopes through either percutaneous or transarterial approach. The ideal conditions for the radioisotopes involve; a short half life and beta ray emitting as a therapeutic purpose but with a little portion of gamma ray for verification. Three types of isotopes have been reported; Yttrium-90 (90Y), a pure beta emitter with a physical half life of 2.7 days, has been attempted by intratumoural injection of 90Y glass microspheres43,44 under ultrasonographic guidance or by transarterial approach. Iodine-131 (131I), mostly beta and a little gamma emission with a half-life of 8 days, has been applied in a form of 131I-lipiodol.45,46 Homium-199 (199Ho), mostly beta and a little gamma emission with a half-life of 26.8 h, has also been tried in chitosan complex form either intratumorally or transarterial approach.47,48 Substantial success has been achieved through these treatment, however, there remain several problems. Unwanted leak of the isotopes may happen through the vascular shunts, which frequently accompany HCC. Dose distribution in and around the tumor also remains to be defined.49 The indication for internal radiotherapy appears to be limited to rather smaller tumors than the advanced ones. Although more discussion will be required regarding internal radiotherapy, this review will focus on external radiotherapy.
External radiotherapy can be applied alone or, more popularly, in combination with chemotherapy. Early trials of external radiotherapy for HCC adopted whole liver radiation of no more than 21 Gy with chemotherapy.21-23 These trials failed to show a therapeutic success, however, it gave a lesson that combination of chemotherapy and radiation may have potential benefit. Current consensus is to treat not the whole but the focal liver with therapeutic dose of radiation.
For small tumors, radiotherapy alone can successfully be performed with high tumoricidal level of radiation dose. In this case, radiation fractionation can be either in conventional or in hypofractionation. A French group performed a prospective phase II trial of conformal radiotherapy.32 Radiotherapy of 66 Gy in conventional fractionation has been done for 27 HCC patients with small tumors which were either single nodule smaller than 5 cm or 2 nodules, each larger than 3 cm. The result was excellent with a response rate of 92%. However, they also experienced substantial toxicity; 19% grade 3 in Child-Pugh score A group and 22% grade 4 in score B group. In other retrospective reports, radiotherapy with higher doses was shown to achieve similar success.16
Small tumors appear to be the best candidates for stereotactic body radiation therapy (SBRT) using hypofractionation. Since the first trial in 1995, SBRT has been performed in various dose fractionation schemes on liver tumors both primary and metastatic.50-52 Canadian group reported a phase I trial for 31 HCC patients. With the median dose 36 Gy (between 24 and 54 Gy) in 6 fractions, they achieved 65% local control rate in 12 months and overall median survival time of 11.7 months.53
Radiotherapy alone can be an effective modality for small tumours. However, in treating locally advanced tumours, radiation doses are limited by liver tolerance, particularly for patients with cirrhosis. In Asia, where TACE has been a major nonsurgical option, radiotherapy has been introduced to improve the high rates of progression after TACE. TACE, using iodized poppy seed oil, Lipiodol and the anticancer drug, has been a popular treatment in Asia. However, its limitation has also been well documented as demonstrated in the pathologic evidence from patients who later underwent resection.54,55 Tumors remain viable particularly in and around the capsule and may recur via the blood supply from collateral circulation or recanalization of the originally embolized artery. Even in encapsulated tumors, which are the favorable tumor type for TACE, the necrosis rate is reported to be no more than 44% when HCCs are larger than 3 cm.56 In locally advanced HCCs, it is almost impossible to achieve a measurable response.
Radiotherapy can effectively ameliorate the limitations of TACE through its antitumor activity as well as anti-vascular activity.57 Radiation may also interact with the chemotherapeutic drug while the drug stays in the tumors after TACE. Adriamycin, injected at the time of TACE, is well known to augment the antitumor efficacy of radiation.58 This drug, when mixed with Lipiodol, has been reported to maintain relatively high concentration in tumors as long as 27 days and decrease to a trace level after 47 days.59,60 In the first report of combination treatment of TACE and radiation by Seong, et al.,12 tumor response rate was 63.3%. Survival rates at 1, 2, and 3 years were 67%, 33.3%, and 22.2%, respectively, with the median survival time of 17 months. Considering the patients' characteristics, all with stages III or IVa with large tumors (mean tumor diameter 8.95 ± 3.4 cm), this regimen seemed to be effective. Radiotherapy has also been attempted to salvage HCC patients who failed with TACE, giving 66.7% response rate and median survival time of 14 months.13
In the retrospective comparative study by the same group, a statistically significant improvement in survival was seen in patients treated with TACE+RT compared to TACE alone (2-year survival rate 37% versus 14% for RT versus repeated TACE, p = 0.001) (Fig. 4).61 The survival difference was greatest for larger tumours. The 2 year survival rates for TACE + radiotherapy and TACE alone were 63% versus 42% in 5-7 cm tumors, 50% versus 0% in 8-10 cm tumors, and 17% versus 0% in tumors larger than 10 cm, respectively.61 Similar results have been shown in other reports.62-65 Recently, Chinese group underwent a metaanalysis questioning the benefit of TACE plus radiotherapy by comparing to TACE alone for unresectable HCC. Through analyzing a total of 1,476 patients from 5 randomized controlled trials and for 17 nonrandomized trials, they concluded that both tumor response and survival outcome were benefited with radiotherapy.66
Combination of chemotherapy and radiotherapy trial has been actively performed by Michigan group.18,67 They performed conformal hyperfractionated radiotherapy (1.5 Gy twice daily over 6 to 8 weeks) with concurrent hepatic arterial floxuridine in serial phase I/II studies of unresectable HCC. In the latest phase II study, the median survival of 15.2 months has been reported in 25 HCC patients treated with doses as high as 90 Gy,28 providing a rationale for high dose local radiotherapy for HCC. They have proposed a practical guideline, suggesting that higher radiation dose can be delivered if the radiation volume is limited to the partial liver. Another approach of concurrent chemoradiotherapy has been performed in stage IVa HCC patients.68 Han, et al.69 reported promising survival results of conformal RT combined with hepatic arterial chemotherapy of 5-FU and cisplatin in 40 patients with locally advanced HCC with portal vein tumour thrombosis (PVTT).69
Table 2 summarizes selective series of radiotherapy in combination with chemotherapy either in TACE or in regional chemotherapy. Some series show promising survival outcome. However, the benefit is obscured due to the nature of mostly retrospective or phase I, II trials and heterogeneity in radiation doses as well as the patient characteristics. Prospective trials are required to confirm the efficacy of chemoradiotherapy.
Association of PVTT in locally advanced HCC has been known to severely limit the therapeutic option, including TACE and poor survival outcome as well.70 In their study, the 3 year overall survival rate was 24.1% and the median survival time was 13.1 months, which was superior the previously reported survivals of 4-6 months. The treatment outcomes have been updated for 101 patients, showing a median survival of 16.7 months (Fig. 5).71 Combination strategies have also been attempted with antiangiogenics by a Japanese group.72 Hyperfractionation RT of 45-75 Gy (1.5 Gy fractions bid) was combined with thalidomide in 121 HCC patients, showing 60% survivals at 1 year and 45% at 2 year.
Different approaches have been attempted for HCC patients with PVTT. Since PVTT is a major obstacle to perform TACE, some groups used radiotherapy for the purpose of opening of PV.73,74 By including only PVTT within a radiation volume, the response rate of PVTT was between 20% and 100%, and complete response was shown around 30%. Other group included PVTT as well as primary tumor within a radiation volume.69,75-78 The response rate as a whole was between 39 to 71.4% with complete response less than 10%. Survival outcome seemed higher in the latter approach. It seems that targeting PVTT only can be useful to open PV in some occasions however, overall outcome seems better with treating both the primary tumor and PVTT. The selected results are summarized in Table 3.
The unique characteristic of charged particle beam, the Bragg peak, allows deposition of high dose of radiation within the target and then sharp dose fall off beyond the target. In addition, charged particle beams do not disperse, which result in sharp lateral margins.79 These characteristics as well as greater radiobiological effectiveness shown in carbon ion beam appear to be fascinating to treat HCC. Tsukuba group in Japan reported the largest series of 162 HCC patients treated with median 72 Gy with or without TAE.80 The results were; the 5 year local control 87% and 5 year survival rate 23.5%. They also showed that repeated proton treatment was safe in selected group of patients. A few more phase II trials have been reported showing that proton beam treatment is safe and effective. However, the advantage of the Bragg peak is somewhat compromised in the process of beam spreading modulation to encompass large tumor volume. Toxicity seems greater than expected with gastrointestinal complication (9%) and hepatic dysfunction (13%).
Carbon ion beam treatment has first been reported by Kato, et al.81 In phase I-II trial of 49.5-79.5 cobalt gray equivalents in 15 fractions for 24 patients, 5 year local control was 81% and 5 year survival was 25%. Particle beam treatment is expected to improve the treatment outcome in the future. However, high cost in purchase, installation and maintenance of hardware is an another problem. Also, technology needs further development to perform perfect intensity modulated particle beam therapy.
Although much progress has been done, there remain many issues unsolved. In this section, some of these are discussed, including optimum dose-fractionation schedule, intra- or extrahepatic metastasis after radiotherapy, and radiation-induced hepatic dysfunction.
In several studies, a radiation dose was shown as a significant prognostic factor in HCC. Dose escalation resulted in increased response rate.24 The radiation dose was also found to be a significant prognostic factor for survival.15,82 Since delivery of tumoricidal dose seems possible if the effective liver volume irradiated is limited to a certain level (i.e., 25%), substantial tumor response can be achievable in selected patients. Importantly, the information of dose-volume statistics through 3-D planning is required as a basic requirement.
Literature survey shows that a variety of fractionation has been adopted; hyperfractionation in b.i.d. schedule, conventional fractionation, and hypofractionation. In retrospective cohort study of Seong, et al.,82 various dose fractionations have been used, ranging from conventional to extreme hypofractionation, using stereotactic technology. Better prognosis was shown in higher doses that have been calculated and analyzed in biological effective dose (Fig. 6).
Escalation of radiation dose brings not only improved tumor response, but also increased toxicity. For example, liver toxicity increased to the double level (from 4.2% to 8.4%) when the radiation dose was escalated from < 40 Gy to > 50 Gy. Gastrointestinal toxicity increased almost to the triple level (from 4.2% to 13.2%) in the same situation.15 Close proximity of radiosensitive gastrointestinal organs is considered as contraindication to this approach.
In determining radiation dose that can safely be delivered, several dose-volume(DVH) guidelines are developed, as shown in Table 1. Briefly, radiation doses are determined by the volume of liver that is irradiated. DVH guideline has also been introduced with additional parameter representing liver reserve function. Cheng et al.83 presented a dose prescription guideline, based on volume and indocyanine green retention test.
Taken together, the best tumor control is expected with higher radiation dose, however, with possible toxicity to liver as well as adjacent gastrointestinal organs. Therefore, consensus is required regarding detailed guideline involving DVH data and other factors that might influence toxicity.
The nature of hepatocellular carcinoma involves presence of vascular invasion in its early stage.19,20 It is also a major contributing mechanism to intrahepatic relapse as well as extrahepatic metastasis to remote organs. It explains why intra- or extrahepatic relapse soon follows after substantial tumor regression by local treatment involving external beam radiotherapy of radiofrequency ablation. Therefore, it seems quite rational to perform combination strategy of local modality and systemic modality.84 Radiotherapy can be combined with transarterial chemoembolization to remedy its weakness, with chemotherapy or with new targeting agents. This approach has been proven to achieve substantial tumor regression even in the advanced disease with accompanying portal vein tumor invasion. A number of novel molecular targeting agents have now been developed and waiting for the results of clinical tests.8,9 Combination of local radiotherapy and novel molecular targeting agents is expected to bring improved results.
Cancer metastasis occurs after incomplete treatment. Even TACE has been reported to enhance metastasis unless complete treatment has been done. For post radiotherapy metastasis, suspicion has been raised for possible enhanced metastasis by radiation. Several reports have suggested molecular mechanisms that radiation might enhance metastasis.85,86 Cheng, et al.85 showed that radiation up-regulates prometastatic molecule, matrix metalloproteinase (MMP)-9. This area of endeavour needs further investigation and ultimate identification of the key molecules will be helpful in improving treatment outcome.
Traditionally, radiation-induced toxicity in liver has been defined as radiation-induced liver disease (RILD), a clinical syndrome of anicteric hepatomegaly, ascits, and elevated liver enzymes (serum alkaline phosphatase) occurring typically 2 weeks to 4 months after radiotherapy. While this concept fits in case of radiotherapy alone, different types of toxicity are frequently seen in combined modality strategy with chemotherapy.36 This entity has been named for combined modality liver disease (CMILD); it presents jaundice and right upper quadrant pain more frequently as well as elevation in bilirubin and serum aspartate transferase.11 CMILD is more lethal with 30-50% mortality. With development of novel targeting agents, combined modality-induced liver toxicity feature might differ from the classic concept. Adoption of general concept "hepatic dysfunction" needs to be seriously considered.
Technical advance as well as deeper understanding of radiobiology increase the use of radiotherapy in management of HCC. Radiotherapy has been proven not only for the palliative benefits for symptomatic patients but also for potentially curative treatment for selected patients by delivering therapeutic dose of radiation in a variety of strategies. Continued progress is being made in technical development as well as newly developed molecular targeting agents, which will bring more opportunity for improved outcome. However, major hepatology societies do not still include this modality in the practice circle for HCC. The most urgent thing to convince these societies is to perform prospective randomized trials to test the efficacy of radiotherapy in HCC. Refinement of radiotherapeutic technology and development of new combination strategy should also be performed in parallel.
Figures and Tables
Fig. 2
Illustration of an exemplary patient treated with TACE plus radiotherapy (54 Gy) for locally advanced HCC. CT scan images are shown for preTACE (A), postTACE (B), postradiotherapy (C), and postresection (D). Note tumor regression as well as compensating hypertrophy of uninvolved liver. No tumor cells were found in surgical specimen. HCC, Hepatocellular carcinoma; TACE, transarterial chemoembolization.

Fig. 3
Illustration of an exemplary patient treated with concurrent radiotherapy (45 Gy) and intraarterial chemotherapy for locally advanced HCC accompanied with portal vein tumor thrombosis. CT scan images of pretreatment (A) and posttreatment (B) are shown. Tumor regression in the primary and portal vein is noted, followed by curative surgical resection (C). HCC, Hepatocellular carcinoma.

Fig. 4
Survival outcome after treatment with either TACE alone (broken line) or TACE plus radiotherapy (solid line) for similar clinical group of HCC patients. The 2 year survival rate appears higher in TACE plus radiotherapy group (36% vs. 14%, p < 0.05, Log-rank test). TACE, transarterial chemoembolization; TACE + RT, transarterial chemoembolization radiotherapy; HCC, Hepatocellular carcinoma.
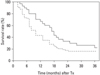
Fig. 5
Survival outcome after concurrent radiotherapy and intraarterial chemotherapy for HCC patients with portal vein tumor thrombosis. Note median survival time of 16.7 months and 2 yr survival rate of 33.7%. HCC, Hepatocellular carcinoma.

Fig. 6
Various dose fractionations in current practice, shown in a national retrospective cohort study. Radiation dose calculated in biologically effective dose (BED) was shown as a significant factor for survival.

ACKNOWLEDGEMENTS
This work was supported by the national R & D program grant for cancer control, the Ministry of Health and Welfare (0620390), by the Korea Science and Engineering Foundation (KOSEF) grant funded by the Korea government (MOST) (2009-0062227), and by a research fund from the Terry Fox Foundation (2008-2009).
References
1. Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005. 55:74–108.

2. Joo KR, Bang SJ, Song BC, Youn KH, Joo YH, Yang SH, et al. Hepatitis B viral markers of Korean adults in the late 1990s: survey data of 70,347 health screenees. Korean J Gastroenterol. 1999. 33:642–652.
3. Bosch FX, Ribes J, Díaz M, Cléries R. Primary liver cancer: worldwide incidence and trends. Gastroenterology. 2004. 127:5 Suppl 1. S5–S16.

4. Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001. 35:421–430.

5. Bruix J, Sherman M. Practice Guidelines Committee. American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology. 2005. 42:1208–1236.

6. Llovet JM, Real MI, Montana X, Planas R, Coll S, Aponte J, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002. 359:1734–1739.

7. Yuen MF, Chan AO, Wong BC, Hui CK, Ooi GC, Tso WK, et al. Transarterial chemoembolization for inoperable, early stage hepatocellular carcinoma in patients with Child-Pugh grade A and B; results of a comparative study in 96 Chinese patients. Am J Gastroenterol. 2003. 98:1181–1185.
8. Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008. 359:378–390.

9. Thomas M. Molecular targeted therapy for hepatocellular carcinoma. J Gastroenterol. 2009. 44:Suppl 19. 136–141.

10. Ingold JA, Reed GB, Kaplan HS, Bagshaw MA. Radiation hepatitis. Am J Roentgenol Radium Ther Nucl Med. 1965. 93:200–208.
11. Lawrence TS, Robertson JM, Anscher MS, Jirtle RL, Ensminger WD, Fajardo LF. Hepatic toxicity resulting from cancer treatment. Int J Radiat Oncol Biol Phys. 1995. 31:1237–1248.

12. Seong J, Keum KC, Han KH, Lee DY, Lee JT, Chon CY, et al. Combined transcatheter arterial chemoembolization and local radiotherapy of unresectable hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 1999. 43:393–397.

13. Seong J, Park HC, Han KH, Lee DY, Lee JT, Chon CY, et al. Local radiotherapy for unresectable hepatocellular carcinoma patients who failed with transcatheter arterial chemoembolization. Int J Radiat Oncol Biol Phys. 2000. 47:1331–1335.

14. Seong J, Park HC, Han KH, Chon CY, Chu SS, Kim GE, et al. Clinical results of 3-dimensional conformal radiotherapy combined with transarterial chemoembolization for hepatocellular carcinoma in the cirrhotic patients. Hepatol Res. 2003. 27:30–35.

15. Seong J, Park HC, Han KH, Chon CY. Clinical results and prognostic factors in radiotherapy for unresectable hepatocellular carcinoma: a retrospective study of 158 patients. Int J Radiat Oncol Biol Phys. 2003. 55:329–336.

16. Park W, Lim DH, Paik SW, Koh KC, Choi MS, Park CK, et al. Local radiotherapy for patients with unresectable hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2005. 61:1143–1150.

17. Cheng JC, Chuang VP, Cheng SH, Huang AT, Lin YM, Cheng TI, et al. Local radiotherapy with or without transcatheter arterial chemoembolization for patients with unresectable hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2000. 47:435–442.

18. Dawson LA, McGinn CJ, Normolle D, Ten Haken RK, Walker S, Ensminger W, et al. Escalated focal liver radiation and concurrent hepatic artery fluorodeoxyuridine for unresectable intrahepatic malignancies. J Clin Oncol. 2000. 18:2210–2218.

19. Masuda T, Beppu T, Ishiko T, Horino K, Baba Y, Mizumoto T, et al. Intrahepatic dissemination of hepatocellular carcinoma after local ablation therapy. J Hepatobiliary Pancreat Surg. 2008. 15:589–595.

20. Matsumoto Y, Fujii H, Matsuda M, Kono H. Multicentric occurrence of hepatocellular carcinoma: diagnosis and clinical significance. J Hepatobiliary Pancreat Surg. 2001. 8:435–440.

21. Cochrane AM, Murray-Lyon IM, Brinkley DM, Williams R. Quadruple chemotherapy versus radiotherapy in treatment of primary hepatocellular carcinoma. Cancer. 1977. 40:609–614.

22. Friedman MA, Volberding PA, Cassidy MJ, Resser KJ, Wasserman TH, Phillips TL. Therapy for hepatocellular cancer with intrahepatic arterial adriamycin and 5-fluorouracil combined with whole-liver irradiation: a Northern California Oncology Group Study. Cancer Treat Rep. 1979. 63:1885–1888.
23. Stillwagon GB, Order SE, Guse C, Klein JL, Leichner PK, Leibel SA, et al. 194 hepatocellular cancers treated by radiation and chemotherapy combinations: toxicity and response: a Radiation Therapy Oncology Group Study. Int J Radiat Oncol Biol Phys. 1989. 17:1223–1229.

24. Park HC, Seong J, Han KH, Chon CY, Moon YM, Suh CO. Dose-response relationship in local radiotherapy for hepatocellulcar carcinoma. Int J Radiat Oncol Biol Phys. 2002. 54:150–155.

25. Tai A, Erickson B, Khater KA, Li XA. Estimate of radiobiologic parameters from clinical data for biologically based treatment planning for liver irradiation. Int J Radiat Oncol Biol Phys. 2008. 70:900–907.

27. Niemierko A, Goitein M. Modeling of normal tissue response to radiation: the critical volume model. Int J Radiat Oncol Biol Phys. 1993. 25:135–145.

28. Ben-Josef E, Normolle D, Ensminger WD, Walker S, Tatro D, Ten Haken RK, et al. Phase II trial of high-dose conformal radiation therapy with concurrent hepatic artery floxuridine for unresectable intrahepatic malignancies. J Clin Oncol. 2005. 23:8739–8747.
29. Seong J, Han KH, Park YN, Nam SH, Kim SH, Keum WS, et al. Lethal hepatic injury by combined treatment of radiation plus chemotherapy in rats with thioacetamide-induced liver cirrhosis. Int J Radiat Oncol Biol Phys. 2003. 57:282–288.
30. Rubin P, Casarett GW. Clinical radiation pathology. 1968. vol 1. Philadelphia: WB Saunders.
31. Emami B, Lyman J, Brown A, Coia L, Goitein M, Munzenrider JE, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991. 21:109–122.

32. Mornex F, Girard N, Beziat C, Kubas A, Khodri M, Trepo C, et al. Feasibility and efficacy of high-dose three-dimensional conformal radiotherapy in cirrhotic patients with small-size hepatocellular carcinoma non-eligible for curative therapies--mature results of the French Phase II RTF-1 trial. Int J Radiat Oncol Biol Phys. 2006. 66:1152–1158.

33. Lee IJ, Seong J, Shim SJ, Han KH. Radiotherapeutic parameters predictive of liver complications induced by liver tumor radiotherapy. Int J Radiat Oncol Biol Phys. 2009. 73:154–158.

34. Xu ZY, Liang SX, Zhu J, Zhu XD, Zhao JD, Lu HJ, et al. Prediction of radiation-induced liver disease by Lyman normal-tissue complication probability model in three-dimensional conformal radiation therapy for primary liver carcinoma. Int J Radiat Oncol Biol Phys. 2006. 65:189–195.

35. Cheng JC, Wu JK, Lee PC, Liu HS, Jian JJ, Lin YM, et al. Biologic susceptibility of hepatocellular carcinoma patients treated with radiotherapy to radiation-induced liver disease. Int J Radiat Oncol Biol Phys. 2004. 60:1502–1509.

36. Dawson LA, Normolle D, Balter JM, McGinn CJ, Lawrence TS, Ten Haken RK. Analysis of radiation-induced liver disease using the Lyman NTCP model. Int J Radiat Oncol Biol Phys. 2002. 53:810–821.

37. Dawson LA, Ten Haken RK, Lawrence TS. Partial irradiation of the liver. Semin Radiat Oncol. 2001. 11:240–246.

38. Shirato H, Shimizu S, Shimizu T, Nishioka T, Miyasaka K. Real-time tumour-tracking radiotherapy. Lancet. 1999. 353:1331–1332.
39. Case RB, Sonke JJ, Moseley DJ, Kim J, Brock KK, Dawson LD. Inter- and intrafraction variability in liver position in non-breath-hold stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys. 2009. 75:302–308.
40. Balter JM, Brock KK, Litzenberg DW, McShan DL, Lawrence TS, Ten Haken R, et al. Daily targeting of intrahepatic tumors for radiotherapy. Int J Radiat Oncol Biol Phys. 2002. 52:266–271.
42. Murphy MJ. Tracking moving organs in real time. Semin Radiat Oncol. 2004. 14:91–100.
43. Dancey JE, Shepherd FA, Paul K, Sniderman KW, Houle S, Gabrys J, et al. Treatment of nonresectable hepatocellular carcinoma with intrahepatic 90Y-microspheres. J Nucl Med. 2000. 41:1673–1681.
44. Geschwind JF, Salem R, Carr BI, Soulen MC, Thurston KG, Goin KA, et al. Yttrium-90 microspheres for the treatment of hepatocellular carcinoma. Gastroenterology. 2004. 127:S194–S205.

45. Order S, Pajak T, Leibel S, Asbell S, Leichner P, Ettinger D, et al. A randomized prospective trial comparing full dose chemotherapy to 131I antiferritin: an RTOG study. Int J Radiat Oncol Biol Phys. 1991. 20:953–963.
46. Raoul JL, Guyader D, Bretagne JF, Heautot JF, Duvauferrier R, Bourguet P, et al. Prospective randomized trial of chemoembolization versus intra-arterial injection of 131I-labeled-iodized oil in the treatment of hepatocellular carcinoma. Hepatology. 1997. 26:1156–1161.

47. Kim JK, Han KH, Lee JT, Paik YH, Ahn SH, Lee JD, et al. Long-term clinical outcome of phase IIb clinical trial of percutaneous injection with holmium-166/chitosan complex (Milican) for the treatment of small hepatocellular carcinoma. Clin Cancer Res. 2006. 12:543–548.

48. Sohn JH, Choi HJ, Lee JT, Lee JD, Kim JH, Moon YM, et al. Phase II study of transarterial holmium-166-chitosan complex treatment in patients with a single, large hepatocellular carcinoma. Oncology. 2009. 76:1–9.

49. Ho S, Lau WY, Leung TW, Chan M, Johnson PJ, Li AK. Clinical evaluation of the partition model for estimating radiation doses from yttrium-90 microspheres in the treatment of hepatic cancer. Eur J Nucl Med. 1997. 24:293–298.

50. Blomgren H, Lax I, Näslund I, Svanström R. Stereotactic high dose fraction radiation therapy of extracranial tumors using an accelerator. Clinical experience of the first thirty-one patients. Acta Oncol. 1995. 34:861–870.

51. Wulf J, Guckenberger M, Haedinger U, Oppitz U, Mueller G, Baier K, et al. Stereotactic radiotherapy of primary liver cancer and hepatic metastases. Acta Oncol. 2006. 45:838–847.

52. Méndez Romero A, Wunderink W, Hussain SM, De Pooter JA, Heijmen BJ, Nowak PC, et al. Stereotactic body radiation therapy for primary and metastatic liver tumors: a single institution phase i-ii study. Acta Oncol. 2006. 45:831–837.

53. Tse RV, Hawkins M, Lockwood G, Kim JJ, Cummings B, Knox J, et al. Phase I study of individualized stereotactic body radiotherapy for hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J Clin Oncol. 2008. 26:657–664.

54. Sakurai M, Okamura J, Kuroda C. Transcatheter chemoembolization effective for treating hepatocellular carcinoma. A histopathologic study. Cancer. 1984. 54:387–392.
55. Yu YQ, Xu DB, Zhou XD, Lu JZ, Tang ZY, Mack P. Experience with liver resection after hepatic arterial chemoembolization for hepatocellular carcinoma. Cancer. 1993. 71:62–65.
56. Higuchi T, Kikuchi M, Okazaki M. Hepatocellular carcinoma after transcatheter hepatic arterial embolization. A histopathologic study of 84 resected cases. Cancer. 1994. 73:2259–2267.

57. Baker DG, Krochak RJ. The response of the microvascular system to radiation: a review. Cancer Invest. 1989. 7:287–294.
58. Byfield JE, Lynch M, Kulhanian F, Chan PY. Cellular effects of combined adriamycin and x-irradiation in human tumor cells. Int J Cancer. 1977. 19:194–204.

59. Nakamura H, Hashimoto T, Oi H, Sawada S. Transcatheter oily chemoembolization of hepatocellular carcinoma. Radiology. 1989. 170:783–786.

60. Raoul JL, Heresbach D, Bretagne JF, Ferrer DB, Duvauferrier R, Bourguet P, et al. Chemoembolization of hepatocellular carcinomas. A Study of the biodistribution and pharmacokinetics of doxorubicin. Cancer. 1992. 70:585–590.

61. Shim SJ, Seong J, Han KH, Chon CY, Suh CO, Lee JT. Local radiotherapy as a complement to incomplete transcatheter arterial chemoembolization in locally advanced hepatocellular carcinoma. Liver Int. 2005. 25:1189–1196.
62. Yasuda S, Ito H, Yoshikawa M, Shinozaki M, Goto N, Fujimoto H, et al. Radiotherapy for large hepatocellular carcinoma combined with transcatheter arterial embolization and percutaneous ethanol injection therapy. Int J Oncol. 1999. 15:467–473.

63. Guo WJ, Yu EX. Evaluation of combined therapy with chemoembolization and irradiation for large hepatocellular carcinoma. Br J Radiol. 2000. 73:1091–1097.

64. Chia-Hsien Cheng J, Chuang VP, Cheng SH, Lin YM, Cheng TI, Yang PS, et al. Unresectable hepatocellular carcinoma treated with radiotherapy and/or chemoembolization. Int J Cancer. 2001. 96:243–252.
65. Zeng ZC, Tang ZY, Fan J, Zhou J, Qin LX, Ye SL, et al. A comparison of chemoembolization combination with and without radiotherapy for unresectable hepatocellular carcinoma. Cancer J. 2004. 10:307–316.
66. Meng MB, Cui YL, Lu Y, She B, Chen Y, Guan YS, et al. Transcatheter arterial chemoembolization in combination with radiotherapy of unresectable hepatocellular carcinoma: a systematic review and meta-analysis. Radiother Oncol. 2009. 92:184–194.
67. Robertson JM, Lawrence TS, Dworzanin LM, Andrews JC, Walker S, Kessler ML, et al. Treatment of primary hepatobiliary cancers with conformal radiation therapy and regional chemotherapy. J Clin Oncol. 1993. 11:1286–1293.

68. Kim JS, Han KH, Lee DY, Seong JS, Youn YH, Cheong JY, et al. Concurrent chemoradiation therapy for advanced hepatocellular carcinoma with portal vein thrombosis. Korean J Hepatol. 2002. 8:71–79.
69. Han KH, Seong J, Kim JK, Ahn SH, Lee do Y, Chon CY. Pilot clinical trial of localized concurrent chemoradiation therapy for locally advanced hepatocellular carcinoma with portal vein thrombosis. Cancer. 2008. 113:995–1003.

70. Tandon P, Garcia-Tsao G. Prognostic indicators in hepatocellular carcinoma: a systematic review of 72 studies. Liver Int. 2009. 29:502–510.

71. Kim JK, Kim MN, Han KH, Seong JS, Lee DY, Lee JT, et al. Prospective large scaled trial of concurrent intraarterial chemoradiation therapy following repeated intraarterial chemotherapy for advanced hepatocellular carcinoma with portal vein thrombosis. J Hepatol. 2008. 48:S148.
72. Hsu WC, Chan SC, Ting LL, Chung NN, Wang PM, Ying KS, et al. Results of three-dimensional conformal radiotherapy and thalidomide for advanced hepatocellular carcinoma. Jpn J Clin Oncol. 2006. 36:93–99.
73. Zeng ZC, Fan J, Tang ZY, Zhou J, Qin LX, Wang JH, et al. A comparison of treatment combinations with and without radiotherapy for hepatocellular carcinoma with portal vein and/or inferior vena cava tumor thrombus. Int J Radiat Oncol Biol Phys. 2005. 61:432–443.

74. Nakagawa K, Yamashita H, Shiraishi K, Nakamura N, Tago M, Igaki H, et al. Radiation therapy for portal venous invasion by hepatocellular carcinoma. World J Gastroenterol. 2005. 11:7237–7241.

75. You CR, Jang JW, Kang SH, Bae SH, Choi JY, Yoon SK, et al. [Efficacy of transarterial chemolipiodolization with or without 3-dimensional conformal radiotherapy for huge HCC with portal vein tumor thrombosis]. Korean J Hepatol. 2007. 13:378–386.

76. Kim TH, Kim DY, Park JW, Kim YI, Kim SH, Park HS, et al. Three-dimensional conformal radiotherapy of unresectable hepatocellular carcinoma patients for whom transcatheter arterial chemoembolization was ineffective or unsuitable. Am J Clin Oncol. 2006. 29:568–575.

77. Kim DY, Park W, Lim DH, Lee JH, Yoo BC, Paik SW, et al. Three-dimensional conformal radiotherapy for portal vein thrombosis of hepatocellular carcinoma. Cancer. 2005. 103:2419–2426.
78. Kim JS, Han KH, Lee DY, Seong JS, Youn YH, Cheong JY, et al. [Concurrent chemo-radiation therapy for advanced hepatocellular carcinoma with portal vein thrombosis]. Taehan Kan Hakhoe Chi. 2002. 8:71–79.
79. Schulz-Ertner D, Tsujii H. Particle radiation therapy using proton and heavier ion beams. J Clin Oncol. 2007. 25:953–964.
80. Kawashima M, Furuse J, Nishio T, Konishi M, Ishii H, Kinoshita T, et al. Phase II study of radiotherapy employing proton beam for hepatocellular carcinoma. J Clin Oncol. 2005. 23:1839–1846.
81. Kato H, Tsujii H, Miyamoto T, Mizoe JE, Kamada T, Tsuji H, et al. Results of the first prospective study of carbon ion radiotherapy for hepatocellular carcinoma with liver cirrhosis. Int J Radiat Oncol Biol Phys. 2004. 59:1468–1476.
82. Seong J, Lee IJ, Shim SJ, Lim do H, Kim TH, Kim JH, et al. A multicenter retrospective cohort study of practice patterns and clinical outcome on radiotherapy for hepatocellular carcinoma in Korea. Liver Int. 2009. 29:147–152.

83. Cheng SH, Lin YM, Chuang VP, Yang PS, Cheng JC, Huang AT, et al. A pilot study of three-dimensional conformal radiotherapy in unresectable hepatocellular carcinoma. J Gastroenterol Hepatol. 1999. 14:1025–1033.

84. Han KH, Lee JT, Seong J. Treatment of non-resectable hepatocellular carcinoma. J Gastroenterol Hepatol. 2002. 17:Suppl 3. S424–S427.

85. Cheng JC, Chou CH, Kuo ML, Hsieh CY. Radiation-enhanced hepatocellular carcinoma cell invasion with MMP-9 expression through PI3K/Akt/NF-kappaB signal transduction pathway. Oncogene. 2006. 25:7009–7018.

86. Jiang Y, Xu W, Lu J, He F, Yang X. Invasiveness of hepatocellular carcinoma cell lines: contribution of hepatocyte growth factor, c-met, and transcription factor Ets-1. Biochem Biophys Res Commun. 2001. 286:1123–1130.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download