Abstract
Moyamoya disease is a cerebrovascular disorder of unknown cause, characterized by slowly progressive bilateral stenosis or occlusion of the internal carotid arteries and produces collateral vessels. Moyamoya syndrome has rarely been reported in association with Graves' disease, especially in children. Several reports suggest that a cerebral infarction might have occurred in patients with clinical and laboratory evidence of hyperthyroid function. We report a case of Moyamoya disease in a girl with Down syndrome and thyrotoxicosis, and we review the relevant literature. To our best knowledge, this is the first report of Moyamoya disease associated with thyrotoxicosis in a young person in Korea.
Moyamoya disease is a cerebrovascular disease with progressive occlusion starting from both internal carotid arteries (ICAs) and extending to their branches, with concurrent formation of a new vascular network at the base of the brain. The typical clinical features are cerebral infarction, recurrent transient ischemic attacks, convulsions and migraine-like headaches in pediatric patients. Although the incidence is relatively high in Japan, it has also been reported in Western countries.1
The pathogenesis of Moyamoya disease is unknown, but hyperactivity of the cervical sympathetic nerves and autoimmune mechanisms observed in thyrotoxicosis may contribute to stenosis of the cerebral arteries.2,3 Although a few cases with concurrence of Graves' disease and Moyamoya disease have been reported,2 a question of whether this leads to an "aggressive autoimmune mechanism" remains to be settled.2,4 Excess thyroid hormone in patients with Graves' disease is harmful to arterial walls as it may alter vascular reactivity,5,6 and Graves' disease is often accompanied by many cardiovascular symptoms.7
The diagnosis of a definite case of Moyamoya disease requires fulfillment of the following angiographic criteria: stenosis or occlusion in the terminal portion of the ICA and in the proximal portions of the anterior cerebral artery (ACA) and the middle cerebral artery (MCA); an abnormal vascular network in the vicinity of the arterial occlusion, and indications of bilateral involvement.8 The idiopathic or primary form of Moyamoya disease has to be distinguished from the secondary form, referred to as Moyamoya syndrome. This syndrome can be associated with certain systemic conditions such as sickle cell disease, chronic basilar meningitis, neurofibromatosis, X-ray irradiation, homocysteinuria and Down syndrome.9
We report a case of Moyamoya disease in a young woman with Down syndrome and thyrotoxicosis. We also review relevant literature and discuss the association of Moyamoya disease with Graves' disease.
A 19-year-old woman who had suffered from Graves' disease for four years visited our outpatient clinic with central type right facial palsy. She also had episodic transient right-side hemiparesis. She had been diagnosed with Down syndrome when she was born. Four years before, she had been admitted to our hospital with diarrhea and a neck mass. Initial thyroid function testing revealed that the thyroid-stimulating hormone (TSH) level was 0.001 microunits/mL (reference range, 0.2 - 7.6 microunits/mL), the triiodothyronine (T3) level was 681 ng/dL (reference range, 80 - 210 ng/dL), and the free thyroxine (free T4) level was over 6.0 ng/dL (reference range, 0.7 - 1.7 mg/dL). Thyroid aspiration study was consistent with diffuse hyperplasia, and the patient had also been diagnosed with Graves' disease. After treatment with propylthiouracil, her hyperthyroid symptoms were controlled, with her thyroid hormone levels maintained at the upper range of normal. Three years after the treatment for hyperthyroidism, she suddenly developed weakness in the right upper and lower extremities. She had grade 3 power in the upper extremity flexors and extensors, and grade 4 in the lower extremity. The biceps and knee jerk reflexes on the right side were hypereflexic. We tried to check her brain using magnetic resonance imaging (MRI), but failed because she was uncooperative and not sedated. The weakness was prolonged for one month, and it then resolved gradually. One year later, the patient developed right hemiparesis again. There was no sign of sensory involvement or coordination abnormalities. At this time, we could perform a brain MRI. The physical examination at the time of admission revealed bilateral exophthalmos and a goiter. The patient's blood pressure was 135/78 mmHg and her heart rate was 91 beats/min. Electrocardiography demonstrated a regular sinus rhythm, and echocardiographic results were normal. Thyroid function tests revealed thyrotoxicosis, with a TSH level of 0.13 microunits/mL, a T3 level of 221.9 ng/dL and a free T4 level of 2.46 ng/dL. The TSH receptor antibody value was 40.09 IU/L (reference range, negative < 1.0 IU/L, positive > 2.0 IU/L). Other laboratory tests, including blood cell count, prothrombin time and activated partial thromboplastin time, yielded normal results.
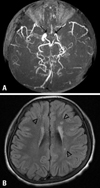
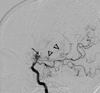
The neurological examination demonstrated right-side hemiparesis. The patient showed alert mentality, bidirectional nystagmus, slurred speech and decreased motor power of grade 4 on the right side. MRI of the brain revealed bilateral stenosis of the sylvian cisternal MCA and intracavernous ICA, prominent especially on the left; there was a multiple chronic infarction in the left side deep periventricular white matter (Fig. 1). Angiography demonstrated bilateral occlusion of the ICA around the supraclinoid segment with some collateral vessels (Moyamoya vessels). The ACA and MCA were not visible, and there were transdural collaterals from both middle meningeal arteries and anterior falx artery to both medial frontal regions (Fig. 2). The patient was diagnosed with Moyamoya disease with typical stenosis of the ICA and MCA, based on MRI scans and cerebral angiography. Single photon emission computed tomographic (SPECT) scans revealed cerebral hypoperfusion of the left frontal, left superior parietal, right superior anterior frontal and anterior cingulate cortices. The patient underwent cranial revascularization by encephalo-duroarterio-synangiosis (EDAS) and encephalo-myo-synangiosis (EMS). A left-side EDAS was done first. Because the size of the right superficial temporal artery was not appropriate for EDAS, a right EMS (application of a temporal muscle flap directly to the pial surface) was done four months later. After surgery, the patient's symptoms improved, and the power of the right upper and lower extremities recovered to grade 5. There has been no recurrence for two years since the last operation. Follow-up angiography was performed six months after surgery and demonstrated that both cerebral hemispheres were well perfused by new transdural collateral vessels.
Excess thyroid hormone in patients with Graves' disease is harmful to arterial walls because it may alter vascular reactivity,5,6 and Graves' disease is often accompanied by many cardiovascular symptoms.
Thyroid hormone directly affects cardiac myocytes and endothelial and vascular smooth muscle cells.10 Hyperthyroidism increases heart rate, cardiac output, pulse pressure and blood pressure.11 The beta index of stiffness provides a useful diagnostic indicator for detecting asymptomatic common carotid artery atherosclerosis and correlates with the severity of atherosclerosis. The beta index of stiffness measured at the common carotid artery in hyperthyroid patients with Graves' disease is increased significantly, which is thought to be due to the increased stroke volume found in these patients.11 Shargorodsky et al.12 showed that patients with this condition have lower large artery elasticity than euthyroid control subjects. Hyperthyroidism is associated with a hyperdynamic circulation, reduced vascular stiffness and enhanced endothelial function.11 Demonstration of increased nitric oxide production and relaxation of vascular smooth muscle in various studies of hyperthyroidism supports these mechanisms.11 In our patient, the duration of diagnosed thyrotoxicosis was four years; however, she might already have had subclinical hyperthyroidism, because she showed exophthalmos at the first visit. There are insufficient data regarding arterial function in patients with subclinical hyperthyroidism, and the evidence for vascular abnormalities in such cases is unconvincing.11 We speculate that this patient might have had vascular alterations due to prolonged subclinical hyperthyroidism.
There have been few reported cases of Graves' disease associated with Moyamoya disease or Moyamoya variants, including our case (Table 1).2-4,8,13-18 Both clinical observations and laboratory evidence of a hyperthyroid state are associated with the onset of cerebral ischemic events, and most of the patients recover from their neurologic symptoms after completion of medical and/or surgical treatment.
According to a study on the stiffness of common carotid artery in patients with Graves' disease, there were strong correlations between all stiffness parameters and plasma thyroid hormone concentrations in the untreated patients, and this relationship remained significant after statistical corrections for age and lipid levels. The stiffness of the common carotid artery in untreated patients might be affected by the thyroid hormone level.7 Some of these patients fared well with the use of antithyroid drugs and the treatment for hyperthyroidism coincided with neurological improvements. Therefore, Moyamoya disease might be asymptomatic, and the cerebral hemodynamic changes attributable to thyrotoxicosis might be the trigger for vascular attacks, either directly or indirectly. It is practical to consider patients with both multiple arterial stenoses and Graves' disease as being at risk for ischemic cerebrovascular events. Sudden surges of thyroid hormone level should be avoided, particularly when using radioactive iodine ablation, which may be more prone to cause these surges.4 Careful control of thyroid function is essential, and surgical revascularization might be considered for selected patients.
Another possible link between these disorders is atherosclerosis. The positive correlation between free T4 levels and both homocysteine and methylmalonic acid indicates the possibility of thyrotoxicosis to induce hyperhomocysteinemia.19 As homocysteinemia has been implicated in patients with atherosclerotic and embolic disorders,20 this could be an another key to the pathophysiology of Moyamoya disease. Graves' disease is an autoimmune disorder, and the associated thyrotoxicosis is thought to enhance the effects of sympathetic nervous activity. Regional sympathetic nervous stimulation may contribute to pathological changes, involving the carotid arteries. This may be because the superior cervical ganglion is adjacent not just to the cervical lymph nodes but also to the thyroid gland.
Arterial stiffness in the common carotid artery is increased in patients with hyperthyroidism. The significant increase in stiffness beta in the hyperthyroid state may reflect the harmful effect of hyperthyroidism on the arterial wall, which may in turn result from increased stroke volume.6 One possible pathomechanism for the aggravation of cerebrovascular ischemia in the thyrotoxic state might be a hemodynamic compromise, induced by an excessive increase in the cerebral metabolism and oxygen demand, exceeding the compensation of the cerebral blood flow deficit through collateral supply in patients with Moyamoya disease.21 Therefore, Graves' disease could be associated with the causal mechanism of Moyamoya disease.
Other disorders associated with Moyamoya syndrome have well-established autoimmune mechanisms of pathogenesis or are associated with arteritis that affects the cerebral arteries. Such diseases include systemic lupus erythematosus, antiphospholipid syndrome, ulcerative colitis, tuberculosis, leptospirosis and postradiation arteritis. Even seemingly nonvasculitic diseases might have an autoimmune etiologic linkage, as suggested for patients with Down syndrome, which has long been known to be associated with Moyamoya syndrome.4 Our patient was diagnosed with Graves' disease and Down syndrome, and was simultaneously diagnosed with Moyamoya disease.
Here, we report a case of Moyamoya disease in a young woman with Down syndrome with thyrotoxicosis. We suggest that strict control of thyroid function in patients with hyperthyroidism might help prevent the associated vascular abnormalities. To our knowledge, this is the first report of Moyamoya disease associated with thyrotoxicosis in a young person in Korea.
Figures and Tables
Fig. 1
(A) Magnetic resonance imaging scans showing bilateral stenosis of MCA and ICA (↑). (B) It is prominent on the left side. Multiple chronic infarction on the left deep periventricular white matter (▵).
References
1. Numaguchi Y, Gonzalez CF, Davis PC, Monajati A, Afshani E, Chang J, et al. Moyamoya disease in the United States. Clin Neurol Neurosurg. 1997. 99:Suppl 2. S26–S30.

2. Tendler BE, Shoukri K, Malchoff C, MacGillivray D, Duckrow R, Talmadge T, et al. Concurrence of Graves' disease and dysplastic
cerebral blood vessels of the Moyamoya variety. Thyroid. 1997. 7:625–629.

3. Kushima K, Satoh Y, Ban Y, Taniyama M, Ito K, Sugita K. Graves' thyrotoxicosis and Moyamoya disease. Can J Neurol Sci. 1991. 18:140–142.

4. Hsu SW, Chaloupka JC, Fattal D. Rapidly progressive fatal bihemispheric infarction secondary to Moyamoya syndrome in association with Graves thyrotoxicosis. AJNR Am J Neuroradiol. 2006. 27:643–647.
5. Honda H, Iwata T, Mochizuki T, Kogo H. Changes in vascular reactivity induced by acute hyperthyroidism in isolated rat aortae. Gen Pharmacol. 2000. 34:429–434.
6. Inaba M, Henmi Y, Kumeda Y, Ueda M, Nagata M, Emoto M, et al. Increased stiffness in common carotid artery in hyperthyroid
Graves' disease patients. Biomed Pharmacother. 2002. 56:241–246.

7. Czarkowski M, Hilgertner L, Powałowski T, Radomski D. The stiffness of the common carotid artery in patients with Graves' disease. Int Angiol. 2002. 21:152–157.
8. Nakamura K, Yanaka K, Ihara S, Nose T. Multiple intracranial arterial stenoses around circle of Willis in association with Graves' disease: report of two cases. Neurosurgery. 2003. 53:1210–1214.
9. Cramer SC, Robertson RL, Dooling EC, Scoutt RM. Moyamoya and Down syndrome. Clinical and radiological features. Stroke. 1996. 27:2131–2135.
10. Ojamaa K, Klemperer JD, Klein I. Acute effects of thyroid hormone on vascular smooth muscle. Thyroid. 1996. 6:505–512.

11. Owen PJ, Sabit R, Lazarus JH. Thyroid disease and vascular function. Thyroid. 2007. 17:519–524.
12. Shargorodsky M, Serov S, Gavish D, Leibovitz E, Harpaz D, Zimlichman R. Long term thyrotropin-suppressive therapy with levothyroxineimpairs small and large artery elasticity and increases left ventricular mass in patients with thyroid carcinoma. Thyroid. 2006. 16:381–386.
13. Liu JS, Juo SH, Chen WH, Chang YY, Chen SS. A case of Graves' disease associated with intracranial moyamoya vessels and tubular stenosis of extracranial internal carotid arteries. J Formos Med Assoc. 1994. 93:806–809.
14. Leno C, Mateo I, Cid C, Berciano J, Sedano C. Autoimmunity in Down's syndrome: another possible mechanism of Moyamoya disease. Stroke. 1998. 29:868–869.

15. Kim JY, Kim BS, Kang JH. Dilated cardiomyopathy in thyrotoxicosis and Moyamoya disease. Int J Cardiol. 2001. 80:101–103.

16. Golomb MR, Biller J, Smith JL, Edwards-Brown M, Sanchez JC, Nebesio TD, et al. A 10-year-old girl with coexistent moyamoya disease and Graves' disease. J Child Neurol. 2005. 20:620–624.

17. Sasaki T, Nogawa S, Amano T. Co-morbidity of moyamoya disease with Graves' disease. report of three cases and a review of the literature. Intern Med. 2006. 45:649–653.

18. Tsai MH, Tan TY, Kuo YL, Chang KC. Multiple intracranial arterial stenoses in association with thyrotoxicosis: a case report. Acta Neurol Taiwan. 2006. 15:105–108.
19. Colleran KM, Ratliff DM, Burge MR. Potential association of thyrotoxicosis with vitamin B and folate deficiencies, resulting in risk for hyperhomocysteinemia and subsequent thromboembolic events. Endocr Pract. 2003. 9:290–295.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download