Abstract
Purpose
Behçet's disease (BD) is a disease of unknown etiology, which has multisystemic involvement. This multisystemic involvement might be the clue for an autoimmune pathogenesis. In order to evaluate an autoimmune pathogenesis, we examined immunoreactans depositions in the skin of BD patients.
Materials and Methods
The skin samples of 108 BD patients (28 perilesional skin, 44 positive pathergy test site, 22 negative pathergy test site, 14 normal skin) were examined for the depositions of immunoglobulin (Ig)M, IgG, IgA, complement 3 (C3), and fibrinogen (F) using direct immunofluorescence (DIF). The data were statistically compared to the DIF of 36 systemic lupus erythematosus (SLE) patients and 20 healthy controls using χ2 Fisher exact test.
Results
Highly significant immunoreactans depositions were obtained in BD (deposition rates: IgM 70.3%, IgG 0%, IgA 20.3%, C3 62.9%, F 83.3%). The comparison with SLE revealed no differences in IgM, IgA, and C3. However, IgG deposition was higher in SLE while F deposition was higher in BD. In both BD and SLE, the Ig depositions were highly significant when the data were compared with the healthy controls.
Conclusion
The significant deposition of immunoreactans in BD, especially in the negative pathergy and the normal skin sites, were observed. This study is the first controlled study revealing positive Ig depositions in BD, and it is expected to help us to reconsider the autoimmune pathogenesis in BD.
Behçet's disease (BD) is a multisystemic disorder with an unknown etiology. Even though autoimmunity is thought to play a part in the etiopathogenesis of BD, there is no clear evidence to support this hypothesis.1 This multisystemic involvement might be a clue for the autoimmune pathogenesis.
The presence of immunoreactans in the skin is either diagnostic or it helps the diagnosis of some autoimmune diseases.2-7 The aim of this study is to evaluate immunoreactans depositions in BD using direct immunofluorescence (DIF), thus implicating the autoimmune theory which we think is important in the etiopathogenesis of the disease. We compared the data of BD both with systemic lupus erythematosus (SLE), which is an autoimmune disease as well as with healthy controls.
A total of 164 skin samples from 108 BD and 36 SLE patients as well as 20 healthy controls were examined for depositions of immunoglobulin (Ig)M, IgG, IgA, complement 3 (C3), and fibrinogen (F) using DIF. The Behçet's Disease Study Group (BD-SG) consisted of 108 randomly selected patients, all fulfilling the International Study Group (ISG) criteria,13 who were admitted to our Behçet's disease clinic between the years 1996 - 2002. There were 65 female and 43 male patients. The mean age was 33.8 years range, (15 - 57 years). The mean duration of the disease was 8.1 years range, (1 - 34 years). The biopsy materials were taken from normal skin, 1 cm away from the defined lesion in all study groups. In the 28 cases with mucocutaneous lesions, the biopsies were preferably performed 1 cm away from the uninvolved skin of sun-unexposed skin lesion. Punch biopsy specimens were obtained from 28 lesional skin site (15 oral aphthae, 9 pseudofolliculitis, 3 Sweet-like eruptions, and 1 erythema nodosum), 44 positive pathergy reaction sites, 22 negative pathergy reaction site, and 14 normal skin site were obtained from the back. The specimens were frozen with Thermo Shandon® cryomatrix frozen specimen embedding medium and 4 µ sections were obtained (CM 1900, Lecia Microsystems GmbH®, Wetzlar, Germany). Fluorescein antihuman (FITC) IgM, IgG, IgA, C3, and F conjugates (Dako Denmark A/S®, Glostrup, Denmark) were used to detect immunoreactans depositions under immunofluorescein microscope (Zeizz_axioskop MC 63®M 35 W, Carl Zeiss MicroImaging GmbH, Jena, Germany) with DIF technique.
One of the two control groups consisted of 36 SLE patient (the systemic lupus study group = SL-SG), selected randomly from the patients referred to the immunology laboratory of our clinic for lupus band test between the years 1996 - 2002. There were 29 female and 7 male patients with a mean age of 34.3 years range, (15 - 67 years). The mean duration of the disease was 7.6 years (1 - 29 years). The second control group consisted of 20 patients who were admitted to our clinic during the same period with lesions of the skin or mouth but no systemic disease was revealed with histopathologic and clinical examination (the healthy study group = H-SG). There were 16 female and 4 male patients with a mean age of 40.6 years range, (10 - 73 years). The samples taken from sun-unexposed normal skin on the back in the SL-SG and H-SG were examined for immunoreactans depositions using DIF. The data of the study groups were compared to each other with χ2 Fisher exact test, and p value was calculated. The sites of depositions with each immunoreactans were examined in all groups.
The 108 cases in BD-SG were classified into two groups during three-month period of time before and after the biopsies were taken, in a total six months period according to the clinical condition as activity remissions. The patients were carefully classified according to criteria indicated below.
Recurrences of the mucocutaneous lesions 3 - 4 times and/or addition of a new type of systemic involvement and/or addition of the mucocutaneous manifestations for the first time and/or continuous arthritis and arthralgias and/or addition of the systemic involvement for the first time in a one-month period of time.
Absence occurrence of the mucocutaneous manifestations 1 - 2 times in a one-month period time and/or shortening of the remissions period of the present mucocutaneous lesions, or disappearing of the symptoms and/or disappearing of the symptoms and signs of current systemic involvement and/or presence of arthralgia(s) attacks for 1 - 2 times.
The 108 cases of BD-SG were compared according to the clinical course of activity or remissions as DIF (+) and DIF (-) using χ2 Fisher exact test. Besides, 108 cases of BD-SG were classified into two groups according to age and gender; the differences between these groups were defined as DIF (+) and DIF (-). χ2 for trend test for age, and χ2 test for gender were used.
The sera of BD-SG and control group patients were not investigated for autoantibodies and immunoreactans.
The clinical findings of the BS-SG are summarized in Table 1. One hundred and eight Behçet's disease patients were enrolled in this study. The SL-SG consisted of 36 patients and the H-SG consisted of 20 patients. The immunoreactans depositions of the BD-SG according to the sample sites and the site of depositions are listed in Table 2 and Table 3, respectively. The depositions of all components were detected, except for IgG. In the BD-SG, the comparison of immunoreactans depositions in different sample sites (perilesional skin, positive and negative pathergy sites, normal skin) revealed no significant difference (Table 2).
When the data of the BD-SG were compared to the data of the SL-SG, the IgG depositions was highly significant for the SL-SG (p < 0.0001), while the F deposition was highly significant for the BD-SG (p < 0.0001). There were no differences in IgM, IgA, and C3 depositions neither in the BD-SG nor in the SL-SG (Table 4).
In the H-SG, nine depositions were detected. Two of them were detected as cytoid bodies with IgM, one of them was a perivascular deposition with F, and six of them were granular deposition of C3 in the dermoepidermal junction.
When the BD-SG was compared to the H-SG, depositions in all of the components were highly significant in the BD-SG. When the SL-SG was compared to the H-SG, the depositions were highly significant in the SL-SG (Table 4).
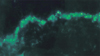
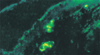
The immunoreactans were deposited either in the perivascular region (P), or in the dermoepidermal junction (DEJ), or in both of them (P + DEJ). In the BD-SG, the most frequent deposition was in the P (84%) which was higher with F than with IgM (Fig. 1). The deposition in the DEJ was in a granular band pattern, only with C3 (9%) (Fig. 2). This deposition in the DEJ was sometimes seen together with the P deposition (7%) (Fig. 3, Table 3).
The highest deposition rate in the SL-SG was in the DEJ with 84.1% (56.8% only in the DEJ, 27.3% together with P deposition).
The distribution of immunoreactants in the active and remitting groups of 108 BD-SG cases were classified according to the material type and are shown in Table 5. There was no statistically significant difference in the 4 different lesion types between the distribution of immunoreactants and activity of the disease (p > 0.05, Table 5).
In this study, immunoreactants depositions were examined in the perilesional skin, pathergy test sites (positive and negative) and normal skin of patients with BD, using DIF. In literatures, it is advised to take the materials from the normal skin. Moreover, the deposition of immunoreactants in the sun-exposed areas has been shown to be more than non-sun-exposed skin.20-22 For this reason, we paid a great deal of attention to this point in the study. However, since the patergy test was applied over the forearms on a sun-exposed area, the results of the immunoreactants depositions obtained from these areas are doubtful. Nevertheless, there is little doubt as the rates of immunoreactants depositions were not statistically different in the other materials (non-lesional skin of Behçet's disease cases and lesional skins). The DIF studies of the BD in the literature are available for us which done on small groups without control groups.8-12
In 1976, Haim et al.8 could not find any depositions with IgA, IgG, IgM, IgE, C3, C4, albumin and fibrinogen in the specimens of 18 BD patients, obtained from the pathergy reaction sites. In 1977, however, Azizlerli et al.9 found depositions in the pathergy test sites of seven BD patients out of eight (4 with IgM, two with IgG, 1 with IgM, IgG, and C3).
In 1981, Luderschmidt et al.10 studied DIF of the oral aphthae of 34 BD patients and found depositions with C3 and C1q in 30 patients, and perivascular deposition with IgM and IgG in 8 patients. The deposition with C3 was reported to be a band-like granular pattern. The author concluded that oral aphtha was an immune complex vasculitis.10 In 1983, Reimer et al.11 studied a group of nine BD patients, and reported C3 deposition in the oral aphthae of the nine BD patients, and IgM depositions in three of them.
In our previous study in 1994, a group of 58 BD patients were examined with DIF and histopathological findings were compared. The samples were taken from perilesional skin, positive and negative pathergy sites, and normal skin. The rates of depositions were 70% for IgM (n = 41), 22.4% for IgA (n = 13), 65.5% for C3 (n = 38), 81% for F (n =47), and vasculitis was found histologically in 52 patients of 58 (89.6%), in which there was a leucocytoclastic type in 70.6% of them. Both DIF and the histopathologic manifestations were in accordance with each other.12
Alpsoy et al. performed a study in 2003 similar to our study in 1994,12 in which they found statistical significance in both IgM depositions (p < 0.05) and histopathologically leucocytoclastic vasculitis (p < 0.05) in the papulopustular lesions.23
Comparing the DIF results of Alpsoy with our 28 BD-SG cases, we obtained deposition of IgM in 20 cases (71.4%) against 52.9% rate reported by Alpsoy. Although we did not found any IgG deposition, Alpsoy et al. found 35.3%.23
It is highly likely that the higher rates of the mucosal lesions (15 cases with aphthous lesions) made this difference, since 9 patients of 28 cases had pseudofollculitis. However, considering the data of the 108 cases, absence of any statistical difference in the skin and the type of the lesion between the immunoreactants depositions weakens this doubt (Table 2).
In the present study, we detected significant immunoreactants depositions in four different lesion sites of our 108 BD patients (Table 2). When the depositions were compared according to the lesion site, no significant difference was detected. Especially, the similar data of depositions in the lesional skin and positive pathergy site, compared to the negative pathergy site and normal skin is an important observation, pointing out possible immunologic mechanisms in the pathogenesis of BD. To our best knowledge, this is the first report that evaluated the immunoreactants depositions in the normal skin and the negative pathergy sites of BD patients.
When the immunoreactants depositions of BD patients were compared to the depositions in the non-sun-exposed, uninvolved skin sites of SLE patients, there was no significant difference in the deposition rates between the two groups (p > 0.05). There were significant depositions with IgM, IgA, and C3 in both groups. Additionally, the F deposition was higher in BD than in SLE (p < 0.0001). Because SLE is an autoimmune disease with well known DIF findings,14-19 the depositions of IgM, IgA, and in C3 BD, which were detected as much as in SLE is suggestive of the immunologic pathogenesis in BD.
The comparison of the results of both BD and SLE groups with the results of healthy controls revealed significant deposition rates in the first two groups (Table 4). This data confirm the finding of immunoreactants depositions in BD, and it is not incidental.
In the literatures, there are no DIF studies performed in healthy cases. Mostly, DIF was performed in normal patient skins. Also in these studies, the rates of depositions in sun-exposed areas were higher than the non-sun-exposed areas.20-22 There was no proven disease based on the clinical findings, except using the immunofluorescence and histopathological analyses in 20 cases (H-SG). The depositions of C3 were found in 6 cases (30%), F in one (5%), and IgM in 2 (10%) of patients. Comparing our data with the control group of follicular acneiform lesions in the study of Alpsoy, we find the most deposition of C3 in 6 cases (30%); although this rate was 17.6% in Alpsoy's study. The IgM and fibrinogen deposition rates were 10% and 5% in our study and 17.6% and 17.6% in Alpsoy's study, respectively. Except C3, the other deposition rates in Alpsoy's study are higher than the ones in our study.23 If we think of technical errors in this study, as it is a controlled research, the same technical error would occur in all groups, therefore, our results seem to be believable.
The autoantibodies in the sera of patients were not detected in this study. If these components were also searched, it would possibly be a better guide in BD etiopathogenesis.
As a result, in this study, the significant deposition of immunoreactants in BD in four different groups lesions (lesional skin, positive and negative pathergy sites, and normal skin) was an important observation. This data were compared to the depositions in SLE patients and healthy controls to find out its significance. Thus, the autoimmune mechanisms in the etiopathogenesis of BD should be reconsidered, even though it currently lost some interest.
Figures and Tables
Table 5
Distribution of the Immunoreactants Depositions according to the Clinical Activation in BD-SG
ACKNOWLEDGEMENTS
The author thanks to G. Azizlerli, R. Sarlca, G. Balcloglu, H. Issever and E. Sagllk for their kind help in this study.
References
1. Yazıcı H, Fresko I, Tunç R, Melikoglu M. Ball GV, Bridges SL, editors. Behçet's syndrome: pathogenesis, clinical manifestations and treatment. Vasculitis. 2002. New York: Oxford University Press;406–432.
2. Laskaris G, Sklavounou A, Angelopoulos A. Direct immunofluorescence in oral lichen planus. Oral Surg Oral Med Oral Pathol. 1982. 53:483–487.

3. Provost TT, Reichlin M. Immunopathologic studies of cutaneous lupus erythematosus. J Clin Immunol. 1988. 8:223–233.

4. Kulthanan K, Roongphiboolsopit P, Chanjanakijskul S, Kullavanijaya P. Chronic discoid lupus erythematosus in Thailand: direct immunofluorescence study. Int J Dermatol. 1996. 35:711–714.

5. Helm KF, Peters MS. Deposition of membrane attack complex in cutaneous lesions of lupus erythematosus. J Am Acad Dermatol. 1993. 28:687–691.

6. Wojnarowska F, Bhogal B, Black MM. The significance of an IgM band at the dermo-epidermal junction. J Cutan Pathol. 1986. 13:359–362.

7. Laskaris G. Oral pemphigus vulgaris: an immunofluorescent study of fifty-eight cases. Oral Surg Oral Med Oral Pathol. 1981. 51:626–631.

8. Haim S, Sobel JD, Friedman-Birnbaum R, Lichtig C. Histohogical and direct immunofluorescence study of cutaneous hyperreactivity in Behçet's disease. Br J Dermatol. 1976. 95:631–636.

9. Azizlerli G, Saylan T, Cologlu AS, Bozan G, Urgancioglu M. Major immunoglobulins in Behçet's disease and a study with direct and indirect immunofluorescent methods. Excepta Medica. 1977. 232–235. International Congress Series.
10. Luderschmidt C, Wolff HH, Scherer R. [Apthae: histologic, immunofluorescent and immuno--electron microscopy study of their pathogenesis.]. Hautarzt. 1981. 32:364–369.
11. Reimer G, Luckner L, Hornstein OP. Direct immunofluorescence in recurrent aphthous ulcers and Behçet's disease. Dermatologica. 1983. 167:293–298.

12. Akdag Kose A, Sarica R, Azizlerli G, Ovül C, et al. Gunes AT, Avcı O, Ozkan S, Fetil , editors. Behçet hastalarında Pategy pozitif, negative, lezyonlu ve lezyonsuz deride histopatolojik ve immunfloresan bulguların karsilastirilması. XV. National Dermatology Congres-III. Internatıonal TURKOD Kurultayi (31.10-4.11.1994 Izmir). 1996. Izmir: Bildiri Kitabı;190–193.
13. International Study Group for Behçet's Disease. Criteria for the diagnosis of Behçet's disease. Lancet. 1990. 335:1078–1080.
14. Akdag KOSE A, Sarica R, Azizlerli G, Ozturk AS, Balcıoglu G, Sur H. The importance of direct immunofluorescence in the diagnosis of lupus erythematosus. Turkderm. 1997. 31:114–116.
15. Weigand DA. Lupus band test: anatomic regional variations in discoid lupus erythematosus. J Am Acad Dermatol. 1986. 14:426–428.

16. Williams RE, Mackie RM, O'Keefe R, Thomson W. The contribution of direct immunofluorescence to the diagnosis of lupus erythematosus. J Cutan Pathol. 1989. 16:122–125.

17. Amital H, Shoenfeld Y. Autoimmunity and autoimmune disease such as systemic lupus erythematosus. 1999. San Diego: Academic Press;1–16.
18. Sontheimer RD. Lahita RG, editor. Systemic lupus erythematosus and the skin. Systemic lupus erythematosus. 1999. San Diego: Academic Press;631–656.
19. Rowell NR, Goolfield MJD. Champion RH, Burton JL, Burns DA, Breathach SM, editors. The connective tissue disease. Textbook of dermatology. 1998. London: Blackwell Science Press;2437–2575.
20. Dahl MV. Usefulness of direct immunofluorescence in patients with lupus erythematosus. Arch Dermatol. 1983. 119:1010–1017.

21. Beutner EH, Chorzelski TP, Jablonska S. Immunufluorescence test. Clinical significance of sera and skin in bullous disease. Int J Dermatol. 1985. 24:405–421.




 PDF
PDF ePub
ePub Citation
Citation Print
Print








 XML Download
XML Download