This article has been corrected. See "Erratum to "Immunoglobulin VH Chain Gene Analysis of Peripheral Blood IgM-Producing B Cells in Patients with Kawasaki Disease" by Lee HH, et al. (Yonsei Med J 50(4):493-504, 2009)." in Volume 50 on page 736.
Abstract
Purpose
Kawasaki disease is a systemic vasculitis, and its etiology and pathogenesis are still not clear. Our study was undertaken to investigate the characteristics of the activation of B cells in the peripheral blood of Kawasaki disease (KD) patients and evidence of stimulation by superantigens.
Materials and Methods
Blood samples were obtained from three patients (2 males, 1 female) with KD, who were admitted to our Hospital, Seoul, Korea. The mean age was 1.2 years. Distribution of B cells was studied in the acute and subacute phases of KD patients. From the RNA of B cells, we obtained complementary DNA (cDNA) and performed polymerase chain reaction (PCR). To determine the oligoclonal expansion of immunoglobulin M (IgM) VH family, we cloned and sequenced the PCR products from each group and analyzed DNA.
Results
In the peripheral blood of acute phase patients, T cells were significantly decreased (p < 0.05), whereas B cells were significantly increased (p < 0.05). When the first PCR was done on the B cell chains, VH1 to VH6 were all found to be expressed. The number of µ gene clones obtained from 3 patients was 312, and they belonged to VH3, VH4 and VH5 family. M99686 germ line was most frequently used and the next most frequently used, were X92224/J, L21967 and L21964. A similar order was seen in patients. Among the clones, 20 sets of clones showed the same base sequence and this was frequent between VH2 and VH5. There was one set, which showed almost the same base sequence between different patients, and the homology was 99.5%. Twenty sets of clones that had the same base sequence showed high similarity to the germ line (94 - 100%). Among these, the clones that utilized the M99686 germ line were 4 sets which were most frequent. The 3-dimensional structure of one of these clones showed typical β, sheet structure of immunoglobulin chains.
Kawasaki disease (KD) is a systemic vasculitis characterized by fever, cervical lymphadenopathy, conjunctival injection, oral lesions, indurative edema, late desquamation of extremities, and polymorphous skin rashes. Complications of KD include coronary artery aneurysms and thrombosis, making it an important cause of acquired heart disease in children.1 Clinical and epidemiological features strongly suggest that the etiology of KD is an infection.2 These include young age of affected individuals range, (6 months to 5 years old), clinical features of the illness such as fever and self-limited course, the occurrence of epidemics with periodicity, and the geographic wave-like spread of illness during epidemics.2
Immunologic mechanisms are thought to be important in disease activation, progression, and complications. The selective expansion of T cells expressing Vβ2 and Vβ8 families in the peripheral blood of acute KD patients has been observed, suggesting the involvement of superantigens.3 Based upon a single study,4 toxic shock syndrome toxin-1 or streptococcal superantigens were proposed to be related etiologically to KD, however, other investigators have not been able to confirm these findings. Over the past few years, it has been debated whether KD is developed as a response to a superantigen or to a conventional antigen.
Rowley et al.5 reported that immunoglobulin (Ig) A produced in acute KD vascular tissue is oligoclonal, consistent with an antigen-driven immune response. In young infants, primary antigenic stimulation of peripheral blood induces B cells to predominantly secrete IgM.6,7 In this study, we examined the clonality of the IgM response in peripheral blood during acute phase of the disease to determine actual immunologic response.
Blood samples were obtained from 3 patients (2 males, 1 female) with KD, who were admitted to our Hospital, Seoul, Korea. All patients satisfied at least 5 of the 6 diagnostic criteria for KD.8 They exhibited typical clinical symptoms and signs of KD, and did not have any complications of coronary artery aneurysm during 6 months after diagnosis. The meanage was 1.2 years (11, 15, and 18 months). All patients were treated with intravenous gammaglobulin in addition to high-dose aspirin. Whole blood was collected during the acute diagnostic phase before the initiation of any therapy and convalescent phase after therapy. Blood samples from the patients were obtained on the first day of diagnosis for each child and again on the 7th day of diagnosis during the convalescent phase. Informed consent was obtained from the parents of the patients included in the study.
Mononuclear cells were isolated by Ficoll-Hypaque density gradient centrifugation, followed by B cell separation using anti-CD19-coated magnetic beads (Dynabeads M450 Pan B; Dynal, Oslo, Norway). Messenger RNA (mRNA) was isolated from 1 × 106 B cells by using the Oligotex Direct mRNA-Kit (Quiagen Inc., Chatsworth, CA, USA), and complementary DNA (cDNA) was prepared with the Superscript Preamplification System (GIBCO BRL, Gaithersburg, MD, USA) and a random hexamer nucleotide mix.
For analysis of VH genes, a set of 5' primers specific for human VH1, VH2, VH3, VH4, VH5, and VH6 gene families in combination with a 3' primer specific for the first exon of the µ constant region were employed (Table 1).9
All polymerase chain reaction (PCR) reactions were performed in a final volume of 100 µL with 20 pmol each of primer, 200 µM dNTP, 2.5 U of Taq DNA Polymerase (Promega, Madison, WI, USA) and 10 µL of 10 × reaction buffer. Amplification was performed in a thermal cycler and consisted of 35 cycles of 1 min denaturation at 95℃, 1 min primer annealing at 58℃, and 1.5 min extension at 72℃, with a final extension of 5 min at 72℃ PCR products were analyzed in a 2% agarose gel, slices containing the specific band of 500 bp were excised, and DNA was purified using QIAquick Gel Extraction Kit (Quiagen).
Cloning of the PCR product was performed using pCR® 2.1-TOPO® kit and One Shot® competent E. coli (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's protocol. After mixing PCR products and using pCR® 2.1-TOPO® vector, ligation was performed for 5 min at room temperature. One Shot® competent E. coli was mixed and incubated for 5 min on ice. Then, SOC culture media were added after heating for 30 sec in a 42℃ water bath. After incubation in selective media containing X-gal (Sigma Co., St. Louis, MO, USA) and ampicillin (100 µg/mL), transformed positive clones were selected. After these clones were cultured in Luria-Bertani media containing ampicillin (100 µg/mL), plasmids were obtained and DNA was quantified using QIAprep® Miniprep kit (Qiagen, Valencia, CA, USA).
After plasmid DNA was mixed with BigDye® terminator mix (PE Applied Biosystems, Foster City, CA, USA) and sequencing primer, DNA was amplified for 10 sec at 96℃, 5 sec at 50℃, and 4 min at 60℃ for 25 cycles. Amplified DNA was washed with ethanol, and sequences were determined with an ABI PRISM® 310. Each sequence was analyzed directionally using M13 forward primer (5'-GTAAAACGAGGCCAG-3') and M13 reverse primer (5'-CAGGAAACAGCTATGAC-3') by Macrogen Co. Ltd (Seoul, Korea). Sequences were compared using DNA sequencing analysis software (PE Applied Biosystems, Wellesley, MA, USA) and DNAssist v 2.0 (Bellville, South Africa). These sequences were also compared with VH complementarity determining regions (CDRs) and human immunoglobulin germ line genes using http://imgt.cines.fr and advanced Blast search program from the Genbank data base (National Center for Biotechnology Information, NIH, Bethesda, MD, USA).
We observed significantly decreased T cells (p < 0.05) and increased B cells (p < 0.05) during the acute phase, when blood samples during the acute and convalescent phase were separately analyzed (Table 2). Serum IgM showed no significant difference during the acute phase (988 ± 218 mg/dL) compared to control group (1046 ± 264 mg/dL). Serum IgM level was increased to 2723 ± 259 mg/dL 24 hours after administration of intravenous gammaglobulin, and the increased level was maintained at 2470 ± 311 mg/dL 7 days thereafter.
Reverse transcription polymerase chain reaction (RT-PCR) was performed to identify oligoclonal expansion of heavy chains in B-cells of peripheral blood during acute phase. As shown in Fig. 1, all heavy chains from VH1 to VH6 were evenly distributed.
The nucleotide and amino acid sequences of a total of 312 clones were analyzed (Table 3). A total of 101 clones from patient #1, 99 clones from patient #2 and 112 clones from patient #3 were analyzed. In all three patients, a preferential usage or deletion of a certain VH family seemed to be unlikely.
Fifty-four out of 312 clones (17.3%) used M99686 germ line gene of the VH5 family which was the most frequently used clone for the 3 patients. The X92224/J of the VH6 family was the second most frequently used (48 out of 312 clones: 15.4%) (Table 4). These findings suggest that there is a clonal relation among the respective B cells.
The VH gene segments were aligned to the germ-line sequence sharing the highest nucleotide identity. In general, both mutated and unmutated VH genes were found to be expressed in IgM-producing B cells of patients with KD. Eighty seven percent (47/54) of M99686 derived clones were more than 98% identical to the germ-line gene at the nucleotide level, and 70.8% (34/48) of X92224/J derived clones displayed greater than 98% homology. Two M99686 derived clones (clone #5-1-6 and clone #5-3-10) and 3 X92224/J derived clones (clone #6-1-20, clone #6-2-14 and clone #6-3-5) displayed extensive somatic mutations, especially in the FR3 region, whereas others showed only a few somatic mutations and some showed nearly identical germ-line. The M99686 derived clones differed in a number of replacement mutations, mainly in CDR2, however, mutations in X92224/J derived clones were distributed more or less randomly over both CDR and FR segments (Figs. 2-7). Interestingly, two clones (clone #5-1-8 and clone #5-3-4) from 2 different patients were almost identical (Fig. 8). These were M99686 derived clones.
The analyzed CDR3 gene segments of M99686 derived clones appeared to differ markedly in length, ranging from 20 to 29 amino acids of 60 - 87 nucleotides (Fig. 3), while the analyzed CDR3 gene segments of X92224/J derived clones appeared to differ markedly in length, ranging from 15 to 34 amino acids of 45 - 102 nucleotides (Fig. 5).
There has been much controversy as to whether a superantigen or conventional antigen plays a role in KD.10,11 Evidence in support of the superantigen theory is that KD is clinically similar to toxin-mediated diseases such as toxic shock syndrome, supported by an increase in peripheral T cells with Vβ2 family T cell receptors (TCR). Furthermore, the isolation of toxin-producing staphylococcus from KD patients gives credence to this theory.4 However, others failed to isolate staphylococcus from KD patients.
In the study on the mechanism of immunoglobulin in the treatment of KD patients, Leucht et al.12 found that B-cells were produced as a response to superantigens, and Duong et al.13 reported that superantigens were the cause of the damage to coronary arteries in mice model of KD. On the other hand, however, many other studies have shown that KD arises from a conventional antigen stimulus rather than a superantigen stimulus. Choi et al.14 studied T cell response to superantigens, and reported clonal expansion of CD8+ T cells to a conventional antigen, however, they could not demonstrate an increase of T cells with Vβ family TCR to a superantigen stimulus in peripheral blood of KD patients.
Post-mortem examination of patients who died of complications from Kawasaki disease showed infiltration of coronary arteries by IgA-producing plasma cells, providing further evidence that KD is due to a conventional antigen stimulus.15 IgA-mediated immune mechanism is thought to be due to an antigen stimulus in which the antigen invades the mucosal barrier of the host. Recently, Rowley et al.16 reported that KD-associated antigen may be present in cytoplasmic inclusion bodies of ciliated bronchial epithelial cells in acute fatal cases. These inclusions would be aggregates of viral proteins and nucleic acids. These findings suggest a common, persistent RNA virus of conventional antigen as the etiological agent of KD.
Though there has been active research going on whether KD is due to a conventional antigen or superantigen through T-cell activation, there are only a few reports on B-cell activation. Barron et al.17 were the first to study the B-cell activation and reported that polyclonal IgM and IgG antibodies were increased. The presence of cytotoxic antibodies against endothelial cells in the sera of KD patients was reported, and these antibodies which react against endothelial antigens are thought to be activated by cytokines.18,19 Kim et al.20 reported that the B-cell clones which contained unusually long (11 amino acid codons) VkIII derived light chain CDR3 regions proliferated significantly in the acute phase of KD and decreased in the convalescent phase. Although they reported the expansion of immunoglobulin light chain clones, however, the study did not demonstrate oligoclonality through sequencing. Interesdemonstrate oligoclonality through sequencing. Interes tingly, in a post-mortem study of KD patients, Rowley et al.5 reported that the plasma cells infiltrating the coronary arteries were mostly IgA and IgM-secreting cells, and that oligoclonal IgA reactivity could be observed, suggesting that KD is caused by conventional antigens rather than superantigens.15
However, when the peripheral blood of infants is stimulated with a polyclonal stimulus, IgM is secreted mostly by B-cells, while there are few IgG and IgA-secreting B-cells.6,7 The increase in IgG and IgA-secreting B-cells is observed at 1-2 years of age. Moreover, the primary immune reaction takes place first when the antigen is introduced into the host, and it is well known that the characteristics of this reaction is that IgM is mostly produced. The affinity and specificity of the antibodies for this antigen is low, and there is little production of memory B-cells. Therefore, before the IgA reaction in the coronary arteries takes place as reported by Rowley et al.,15 a primary immune reaction, in which IgM-secreting B cells are produced with a subsequent class switch to IgA, probably has already taken place in the peripheral blood. If this is true, then the B-cell reaction observed in the peripheral blood in the acute phase of KD could be an IgM reaction. Therefore, IgM secreting B-cells rather than IgA secreting B-cells should be studied. According to Leung et al.,18 a mechanism involved in vasculitis of KD is the presence of cytotoxic anti-endothelial cell antibodies in the serum of patients that are mostly comprised of IgM antibodies. Therefore, in the study of pathogenesis of KD, the clonality of the IgM transcripts which are expressed by the B-cells in the peripheral blood in the acute phase of KD patients is of primary importance.
Similar to the superantigen of T-cells, the superantigen of B-cells binds to the Fab portion of the VH3 immunoglobulin. Staphylococcal protein A (SPA) is a representative B-cell superantigen and binds mostly with IgM, specifically with the FR3 portion, while some bind with the FR1 and the 3' end of the CDR2.21 Among the B cell repertoire in the peripheral blood, the VH3 family is most commonly used, therefore, the VH3 family clones are probably the most numerous. Thus, if there is a superantigen stimulus, the clone that belongs to a specific VH family should increase or significantly decrease. For example, according to the theory of Hillson et al.,21 the VH3 family should comprise most of the cloned IgM transcripts. However, a similar number of VH1 family and VH6 family clones were obtained and significantly decreased number of VH family clones could not be observed.
Furthermore, the germ line genes used among the 314 clones were 17.3% for M99686 derived clones and 15.4% for X92224/J derived clones, showing that there were numerous clones using similar germ line genes. There were slight differences in the most commonly used germ line genes, nevertheless, most patients used similar germ line genes, thus supporting the oligoclonality of B-cells. Also, these 2 clones do not come from the superantigen-stimulated VH3 family, but rather M99686 is a clone of the VH5 family and X92224/J is a clone of the VH6 family. Although the presence of clonally related sequences might be due to simply isolation of the sequence from the same plasma cell by chance, it is very unlikely. An interesting observation was that the sequence of two clones (5-1-8 clone and the 5-3-4 clone) from different patients, showed 99.5% homology, which suggests that the sequence could not have been isolated from the same plasma cell. Therefore, these findings suggest that these KD patients might be from same antigenic stimulus.
Since the B-cell superantigen binds with the FR portion rather than the CDR portion of the immunoglobulin molecule, it is known that few somatic hypermutations occur.22 Generally, the R/S ratio is 2.9 when random mutations occur.23 The R/S ratio is less than 2.9 when selection occurs to maintain the amino acid base sequence and the ratio is more than 2.9 when selection leans towards diversity.24 When there is no change to maintain function, such as the FR of the antibody, the R/S ratio should be less than 1.5. On the other hand, if the R/S ratio of the CDR of the antibody produced in response to an antigen is more than 2.9, this indicates that the clone containing the antigen-binding site with high affinity for the antigen is positively selected.25 In the present study, it was difficult to analyze all the sequences of the clones. Nevertheless, the fact that the R/S ratio of the CDR clones with similar base sequences was more than 2.9 indicated that positive selection took place, providing evidence for selection because of a conventional antigen stimulus. Furthermore, the R/S ratio of the most commonly used germ line genes M99686 and X92224/J was more than 2.9 and the diverse sizes of CDR3 showed that the clonal expansion was due to a conventional antigen stimulus rather than a superantigen stimulus.
In conclusion, we showed that the diverse immunoglobulin somatic mutations that occur in response to an antigen stimulus are due to antibody affinity maturation, and that the B-cell IgM transcripts in the peripheral blood of KD are due to a conventional antigen stimulus rather than a superantigen stimulus, as Rowley et al.16 reported recently. Furthermore, the IgM produced from peripheral B cells in acute KD is oligoclonal, consistent with a conventional antigen-driven immune response.
Figures and Tables
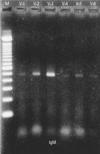 | Fig. 1Amplification of V regions of Ig µ heavy chain genes (VH1 - VH6) by RT-PCR. M, marker; RT-PCR, reverse transcription polymerase chain reaction. |
 | Fig. 2The deduced amino acid sequences of VH germ line gene segments of 54 clones isolated from three different patients are shown in comparison with germ line M99686. |
 | Fig. 3The nucleotide sequences of CDR3 / FR4 and adjacent regions of VH germ line gene segments of 54 clones homologous to M99686 isolated from 3 different patients are shown in comparison with germ line M99686. |
 | Fig. 4Mutation analysis of 54 M99686 derived clones from the peripheral B cells of 3 different patients. |
 | Fig. 5The deduced amino acid sequences of VH germ line gene segments of 54 clones isolated from 3 different patients are shown in comparison with germ line X92224/J. |
 | Fig. 6The nucleotide sequences of CDR3 / FR4 and adjacent regions of VH germ line gene segments of 54 clones homologous to X92224/J isolated from 3 different patients are shown. |
 | Fig. 7Mutation analysis of 48 X92224/J derived clones from peripheral B cells of 3 different patients. |
 | Fig. 8Sequence alignment of 5-1-8 (patient #1) vs. 5-3-4 (patient #3) clones. The sequence of clones showed almost identical alignment. |
References
1. Kawasaki T. Acute febrile mucocutaneous syndrome with lymphoid involvement with specific desquamation of the fingers and toes in children: Clinical observations of 50 cases. Jpn J Allergol. 1967. 16:178–222.
2. Freeman AF, Shulman ST. Recent developments in Kawasaki disease. Curr Opin Infect Dis. 2001. 14:357–361.

3. Abe J, Kotzin BL, Jujo K, Melish ME, Glode MP, Kohsaka T, et al. Selective expansion of T cells expressing T-Cell receptor variable regions V beta 2 and V beta 8 in Kawasaki disease. Proc Natl Acad Sci U S A. 1992. 89:4066–4070.

4. Leung DY, Meissner HC, Fulton DR, Murray DL, Kotzin BL, Schlievert PM. Toxic shock syndrome toxin-secreting Staphylococcus aureus in Kawasaki syndrome. Lancet. 1993. 342:1385–1388.

5. Rowley AH, Eckerley CA, Jäck HM, Shulman ST, Baker SC. IgA plasma cells in vascular tissue of patients with Kawasaki syndrome. J Immunol. 1997. 159:5946–5955.
6. Andersson U. Development of B lymphocyte function in childhood. Acta Paediatr Ascand. 1985. 74:568–573.

7. Andersson U, Bird AG, Britton BS, Palacios R. Humoral and cellular immunity in humans studied at the cell level from birth to two years of age. Immunol Rev. 1981. 57:1–38.

8. Council on Cardiovascular Disease in the Young. Committee on Rheumatic Fever, and Kawasaki Disease. American Heart Association. Diagnostic guidelines for Kawasaki disease. Circulation. 2001. 103:335–336.
9. Brezinschek HP, Brezinschek RI, Lipsky PE. Analysis of the heavy chain repertoire of human peripheral B cells using single-cell polymerase chain reaction. J Immunol. 1995. 155:190–202.
10. Meissner HC, Leung DY. Superantigens, conventional antigens and the etiology of Kawasaki syndrome. Pediatr Infect Dis J. 2000. 19:91–94.

11. Rowley AH. The etiology of Kawasaki disease: superantigen or conventional antigen? Pediatr Infect Dis J. 1999. 18:69–70.

12. Leucht S, Uttenreuther-Fischer MM, Gaedicke G, Fischer P. The B cell superantigen-like interaction of intravenous immunoglobin (IVIG) with Fab fragments of V(H) 3-23 and 3-30/3-30.5 germline gene origin cloned from a patient with Kawasaki disease is enhanced after IVIG therapy. Clin Immunol. 2001. 99:18–29.

13. Duong TT, Silverman ED, Bissessar MV, Yeung RS. Superantigenic activity is responsible for induction of coronary arteritis in mice: an animal model of Kawasaki disease. Int Immunol. 2003. 15:79–89.

14. Choi IH, Chwae YJ, Shim WS, Kim DS, Kwon DH, Kim JD, et al. Clonal expansion of CD8+ T cells in Kawasaki disease. J Immunol. 1997. 159:481–486.
15. Rowley AH, Shulman ST, Spike BT, Mask CA, Baker SC. Oligoclonal IgA response in the vascular wall in acute Kawasaki disease. J Immunol. 2001. 166:1334–1343.

16. Rowley AH, Baker SC, Orenstein JM, Shulman ST. Searching for the cause of Kawasaki disease--cytoplasmic inclusion bodies provide new insight. Nat Rev Microbiol. 2008. 6:394–401.

17. Barron K, DeCunto C, Montalvo J, Orson F, Lewis D. Abnormalities of immunoregulation in Kawasaki syndrome. J Rheumatol. 1988. 15:1243–1249.
18. Leung DY, Geha RS, Newburger JW, Burns JC, Fiers W, Lapierre LA, et al. Two monokines, interleukin 1 and tumor necrosis factor, render cultured vascular endothelial cells susceptible to lysis by antibodies circulating during Kawasaki syndrome. J Exp Med. 1986. 164:1958–1972.

19. Leung DY. The potential role of cytokine-mediated vascular endothelial activation in the pathogenesis of Kawasaki disease. Acta Paediatr Jpn. 1991. 33:739–744.

20. Lee TJ, Kim KH, Chun JK, Kim DS. Low-dose methotrexate therapy for intravenous immunoglobulin-resistant Kawasaki disease. Yonsei Med J. 2008. 49:714–718.

21. Hillson JL, Karr NS, Oppliger IR, Mannik M, Sasso EH. The structural basis of germline-encoded VH3 immunoglobulin binding to staphylococcal protein A. J Exp Med. 1993. 178:331–336.

22. Silverman GJ. Unconventional B-cell antigens and human immune repertoires. Ann N Y Acad Sci. 1995. 764:342–355.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download