INTRODUCTION
Isotretinoin, synthetic vitamin A for severe acne, is a known human teratogen that can cause multiple malformations, including birth defects and intrauterine fetal death. The drug was contraindicated in women who are or may become pregnant during therapy or in the following month, especially within one month. We present a case of ear malformation in a newborn whose mother had taken isotretinoin for 2 years until one month prior to the time when she became pregnant.
CASE REPORT
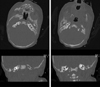
A one-day-old term male neonate was brought to NICU for evaluation of malformation of ear. His mother, a 24-year-old nulliparous woman, had taken 10 mg of Ro-accutane® (isotretinoin) for approximately 2 years due to severe acne. She has used an intrauterine device for contraception during the treatment of Roaccutane®. She was 10 weeks pregnant when she visited the hospital. She had regular 30 day menstruation cycle, and discontinued the use of isotretinoin about 13 weeks ago, meaning that after 5 weeks of discontinuation, fertilization was achieved. She received regular antenatal care during the 1st trimester of pregnancy and there were no noticeable events. Ultrasonographic exam was done only at 10 weeks and 20 weeks of pregnancy, and no definite malformation was confirmed. The baby's birth was uneventful and weighed 3.8 kg at birth. On examination, the baby was conscious and lively. His right ear was deformed and microtic with a visible opening of ear canal, however there was only a skin tag-like remnant of lower part of ear lobe with no opening on the left side (Fig. 1). The other features of HEENT, cardiovascular and CNS systems were within normal limits. Tympanic bone computer tomography showed atresia of bilateral external auditory canal and dilatation of both vestibules (Fig. 2). Brain MRI showed no discernible abnormalities.
DISCUSSION
Accutane®, known by the generic name "isotretinoin," is a prescription oral medication first approved in 1982 by the Food and Drug Administration (FDA) to treat severe, recalcitrant nodular acne. The concern about human teratogenicity proved well founded, because it was soon demonstrated that approximately 25 to 30 percent of exposed fetuses had birth defects - the so-called accutane embryopathy, consisting of craniofacial, heart, and central nervous system defects.1 Malformations frequently observed include microtia/anotia, micrognathia, cleft palate, defects of the auditory canals, facial dysmorphy, heart and aortic arch defects, thymic hypoplasia, microcephaly, hydrocephaly, retina or optic nerve abnormalities, and functional impairment. This issue was taken up and reviewed by an advisory committee to the US FDA in 1988. Following the guidance by the FDA, the manufacturer began a pregnancy-prevention program (PPP) in the same year and developed a new risk management program, the System to Manage Accutane-Related Teratogenicity (SMART), in 2002.2,3 Although the teratogenicity of this drug is well known and risk-management programs had been implemented, preventable fetal exposures have continued, largely as a result of the lack of sufficient controls within the programs themselves.4 The manufacturers of isotretinoin implemented a new risk-management program, iPLEDGE, in March 2006.5 iPLEDGE is a comprehensive distribution system that includes mandatory registration of patients, healthcare providers, pharmacists, and wholesalers. It allows real-time linkage of pregnancy-test results for verification prior to the dispensing of isotretinoin.
There has been numerous reviews about retinoid-induced ear malformation. In in vivo and in vitro studies, isotretinoin interferes directly with the development of cranial neural crest cells.6 Earlier exposure in utero produces microtia, auricular duplication, anotia, temporal bone abnormalities and ossicular malformation. However, an exposure at later developmental stage results in facial tags with less severely affected ears.
The prevelance of microtia has been reported to vary from 0.83 to 17.4/10,000. Microtia has been associated with numerous risk factors such as prenatal exposure to drugs, paternal age, maternal age, high parity, first parity, maternal diabetes, male gender, urban area, altitude, and low birth weight.7 In our case, no typical drug exposure except isotretinoin was found, and no specific history was revealed.
Despite prominent warnings, reports of pregnancies in exposed women continues to accumulate, and malformed infants have been reported. The Slone Survey was an independent follow-up survey of women of childbearing age who were prescribed isotretinoin between January 1989 through June 2000 in which 1,019 exposed pregnancies were identified.3 Of these, 117 live births were recorded and 887 terminations, including 681 elective terminations, 177 spontaneous abortions, and 29 ectopic pregnancies. Of the 63 live births for which records were examined, 13% showed major malformations and 30% showed some degree of malformation.
Malformations may occur even with short periods of use only. Therefore, no systemic dose of isotretinoin is considered safe during pregnancy.7 Hersh et al.8 reported that 10% of live birth records examined showed malformations of pregnancies occurring within 30 days after isotretinoin discontinuation. However, women who conceive one cycle after discontinuing isotretinoin are advised that their teratogenic risk is not higher than baseline.9
The elimination half-life of isotretinoin has been reported to be 10-20 hours, and its metabolites, 4-oxo-isotretinoin, 17 to 50 hours. The pharmacokinetics of isotretinoin (0.47 to 1.7 mg/kg/day) were studied in a small number of childbearing age women, and the results suggested that the half-life may be more variable and/or longer than previously reported, thus affecting the length of time for safe conception after drug discontinuation.10 In one patient who exhibited an abnormally long elimination half-life of the drug , the t1/2 values for isotretinoin and its 4-oxo metabolite were 12.9 hours and 60.75 hours, respectively. The present results confirmed a prolonged t1/2 in a patient, which might have been due to hepatic recirculation. Even in the worst-case scenario (t1/2 of approximately one week), five times of t1/2 s, which are needed to allow levels to return to baseline would be a 35-day period before safe conception.10 According to iPLEGE program, the patient is advised to have a negative pregnancy test before isotretinoin use, every month during treatment, at the end of treatment and 1 month after stopping treatment.
Herein, we found a case of ear malformation occurring after 1 month of isotretinoin discontinuation. We, therefore, suggest that a new guideline recommending a longer duration of isotretinoin discontinuation before conception and further studies of pharmacokinetics isotreinoin are needed.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download