Abstract
In the treatment of recurrent or metastatic gastrointestinal stromal tumors (GIST), good prognoses may not be expected by surgery alone. Recently, imatinib has been applied for the treatment of GISTs, resulting in improved patient survival. However, long-term success is limited due to the development of resistance. Herein, we report two cases of long-surviving patients with recurrent GIST after receiving cytoreductive surgery with imatinib therapy. A 49 year-old man was diagnosed to a duodenal GIST with single hepatic metastasis, and an antrectomy including the duodenal lesion with intraoperative radiofrequency ablation were performed in April, 2002. After four months, a new metastatic hepatic lesion was identified. Percutaneous radiofrequency ablation was done, and imatinib therapy was started. A 56 year-old man underwent laparoscopic segmental resection of the distal ileum and partial excision of parietal peritoneum in March, 2001 to treat a malignant GIST of the distal ileum that was attached to parietal peritoneum. After six months, recurrence of GIST with peritoneal seeding and hepatic metastasis was found, and he underwent cytoreductive surgery including right hemicolectomy and wedge resection of liver. After surgery, there was no residual tumor grossly and imatinib therapy was started. In both cases, they were doing well with no evidence of recurrence for 5 years with imatinib therapy. Therefore, in patients with a recurrent GIST, improved survival can be expected with imatinib therapy after cytoreductive surgery.
For patients with primary, localized gastrointestinal stromal tumors (GIST), surgery with complete excision is the treatment of choice.1 However, more than half of patients experience tumor recurrence during the course of their disease,2 and complete resection can be achieved only in less than half of these patients who had recurrence of the tumors. Recently, imatinib has been applied in the treatment of unresectable or recurrent GISTs, resulting in improved patient survival. However, long-term results of imatinib in metastatic GIST as well as its complete response during treatment are very rare.3-6 Moreover, the results of cytoreductive surgery in patients with far advanced or metastatic GISTs who are treated with imatinib are also rare. Herein, we report two patients who have experienced long-term survival with recurrent GIST after receiving cytoreductive surgery with imatinib therapy.
A 49-year-old man came to the emergency center presenting with epigastric pain and melena in April 2002. Esophagogastroduodenoscopy showed a centrally ulcerative huge mass with bleeding in the superior wall of the duodenal 1st portion. A biopsy was performed, and the mass was confirmed as a spindle cell type GIST with strong c-kit immunoreactivity. A CT scan revealed a 2.5 cm sized solid mass lesion in the duodenum, and a 1.3 cm low-density lesion in the right lobe of the liver (Fig. 1). A liver MRI showed a 1.6 cm sized solitary metastatic nodular lesion in liver segment V. The patient received a distal subtotal gastrectomy with gastroduodenostomy and a negative microscopic surgical margin. The patient simultaneously received intraoperative radiofrequency ablation for the hepatic lesion. Pathological analysis revealed a 3×2 cm sized, well-circumscribed duodenal GIST with an ulcerative surface, and mitotic counts higher than 5 per 50 high-powered fields (HPF) (Fig. 2).
Four months later, a follow-up CT scan was performed, and a new 0.9 cm sized small metastatic lesion was visualized in segment VII of the liver (Fig. 3). Radiofrequency ablation was performed again for the lesion, and the patient was then treated with imatinib mesylate (STI 571, Gleevec® or Glivec™; Novartis Pharmaceuticals, Basel, Switzerland) at a dosage of 400 mg daily. The patient regularly visited our clinic for CT scanning and esophagogastroduodenoscopy. As of April 2007, he was taking the imatinib mesylate, and there was no evidence of tumor recurrence.
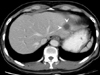
A 56 year-old man was referred to our hospital in September 2001. He had previously received a laparoscopic assisted segmental resection of distal ileum due to a malignant GIST at another hospital in March 2001. The GIST was 6×5 cm in size and attached to the parietal peritoneum. The parietal peritoneum was also excised along with the mass. In April 2001, the patient received a CT scan because of vague abdominal pain. The scan revealed a 3 cm sized solid lesion in the left lateral segment of the liver, and multiple scattered nodular masses in the peritoneal space (Fig. 4). He was diagnosed with recurrence of malignant GIST with peritoneal seeding and hepatic metastasis, and was referred to our hospital for further treatment.
During surgery, several ovoid to spherical congregated masses were found to be attached to the serosal surface of ascending colon and the mesentery of small bowel, omentum, and peritoneal surface. The masses showed smooth, glistening, and multinodular external surfaces, with the diameter of the largest mass at 5 cm. In addition, a 3.5 cm sized mass was found also in the left lateral segment of the liver. Cytoreductive surgery, including right hemicolectomy and wedge resection of the liver, was then performed, and no gross residual tumor remained. Histologically, the tumors in the liver and on the peritoneal surface were confirmed as malignant GIST consisting of spindle cells that exhibited diffuse immunohistochemistry staining for c-kit (Figs. 5 and 6). However, the tumor cells were not stained for smooth muscle actin and the S-100 protein. The patient recovered uneventfully, and he started chemotherapy with imatinib mesylate at a dosage of 400 mg daily after two weeks of surgery. Five years later, the patient was treated with imatinib mesylate and doing well with no evidence of recurrence.
Until recently, the origin and the pathobiology of GISTs were not fully understood, leaving the categorization of such tumors as enigmatic, unpredictable, and rare. As there is no specific staging system or grading for the malignancy of GISTs, it is difficult to predict the metastatic potential for the primary GIST.7 GISTs, except those less than 1.0 cm in size, can exhibit malignant behavior, with the tumor size and mitotic index serving as the most important prognostic factors.8
In primary, localized GISTs, complete resection of the tumors is the treatment of choice.1 However, 20 to 25% of gastric stromal tumors and 40 to 50% of small intestinal stromal tumors undergo metastasis, and more than half of patients experience tumor recurrence during the course of their disease.2 Thus, in the treatment of recurrent or metastatic GISTs, surgery alone is usually not sufficient.
Since 2000, there has been a shift in paradigm for the treatment of GIST, and Kit/PDGFRA tyrosine kinase inhibitors such as imatinib have been applied in the treatment of unresectable or recurrent GISTs. This oral therapy has demonstrated good response in the majority of patients and has has emerged as the gold standard treatment for patients with metastatic GISTs.1 However, long-term success is limited due to the development of imatinib resistance via secondary mutations or clonal selection.9-13 Other inhibitors of Kit/PDGFRA receptors or downstream signaling molecules targets, such as protein kinase theta and tyrosine kinase inhibitors of VEGFRs, have been utilized in cases where imatinib has failed.14,15
Although there are several reports of combination therapy of imatinib with surgery for the advanced GIST with metastases, most of the studies used imatinib neoadjuvantly, and the surgery was performed after the reduction of tumor burden by imatinib therapy. However, we performed the cytoreductive surgery first and then started the imatinib therapy, because complete pathological responses are rare with imatinib therapy alone, and cytoreductive surgery could lessen the risk of recurrence by removing potentially resistant clones.
We tried to complete cytoreductive surgery in two cases of recurrent GIST. The two patients received oral imatinib therapy after cytoreductive surgery, and revealed no residual disease for a long-term period of time. Although more studies are needed to confirm whether cytoreductive surgery is additive to imatinib treatment, our results were very encouraging in this regard. Therefore, in patients with far advanced or recurring GISTs, improved survival could be expected by imatinib oral therapy after removal of tumors as much as possible.
In far advanced or severely metastasized patients, neoadjuvant therapy commonly enables curative surgical resection. Indeed, neoadjuvant therapy with imatinib for treatment of malignant GISTs is able to increase the rate of complete cytoreductive surgery. There are several reports of neoadjuvant imatinib chemotherapy therapy used to treat primary unresectable GISTs, allowing them to be resected completely. However, resistance is commonly observed after neoadjuvant imatinib therapy, and surgical intervention should always be considered in neoadjuvant therapy group, since the duration of neoadjuvant chemotherapy and an optimal time for surgery are decisive factors.16
Even after complete resection of primary tumor, more than half of patients experience recurrence of tumors. And the first site of recurrence is usually limited in the abdomen, even though recurrent GISTs have a multifocal nature.17 However, complete resection can be achieved only in less than half of patients. Thus, patients should be followed up regularly from early period after resection of the primary tumor, because an early detection of metastasis could possibly increase complete cytoreduction of the recurrent tumor burden. Furthermore, this complete cytoreduction followed by imatinib therapy might be able to improve the survival of patients with recurrent GISTs.
Figures and Tables
Fig. 1
Post-contrast CT. About a 1.3 cm round focal lesion with low attenuation density (arrow) showing a target-like appearance of mild contrast enhancement with centrally non-enhancing necrotic area in the right lobe of the liver suggests a single hepatic metastasis.
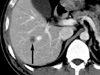
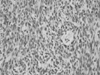
Fig. 2
The histologic picture of duodenal tumor shows the bundles of spindle cells (hematoxylin and eosin staining).

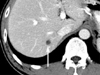
Fig. 3
Four-month follow-up CT shows a newly developed metastasis (arrow) in the posterior segment of right lobe of the liver.
References
1. Gold JS, Dematteo RP. combined surgical and molecular therapy: the gastrointestinal stromal tumor model. Ann Surg. 2006. 244:176–184.
2. Miettinen M, Lasota J. Gastrointestinal stromal tumors: review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch Pathol Lab Med. 2006. 130:1466–1478.
3. van Oosterom AT, Judson IR, Verweij J, Stroobants S, Dumez H, Donato di Paola E, et al. Update of phase I study of imatinib (STI571) in advanced soft tissue sarcomas and gastrointestinal stromal tumors: a report of the EORTC Soft Tissue and Bone Sarcoma Group. Eur J Cancer. 2002. 38:S83–S87.

4. Blanke CD, Rankin C, Demetri GD, Ryan CW, von Mehren M, Benjamin RS, et al. Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. J Clin Oncol. 2008. 26:626–632.

5. Verweij J, Casali PG, Zalcberg J, LeCesne A, Reichardt P, Blay JY, et al. Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomised trial. Lancet. 2004. 364:1127–1134.

6. Blanke CD, Demetri GD, von Mehren M, Heinrich MC, Eisenberg B, Fletcher JA, et al. Long-term results from a randomized phase II trial of standard- versus higher-dose imatinib mesylate for patients with unresectable or metastatic gastrointestinal stromal tumors expressing KIT. J Clin Oncol. 2008. 26:620–625.
7. Ballarini C, Intra M, Ceretti AP, Prestipino F, Bianchi FM, Sparacio F, et al. Gastrointestinal stromal tumors: a "benign" tumor with hepatic metastasis after 11 years. Tumori. 1998. 84:78–81.

8. Miettinen M, El-Rifai W, H L Sobin L, Lasota J. Evaluation of malignancy and prognosis of gastrointestinal stromal tumors: a review. Hum Pathol. 2002. 33:478–483.
9. Tamborini E, Bonadiman L, Greco A, Albertini V, Negri T, Gronchi A, et al. A new mutation in the KIT ATP pocket causes acquired resistance to imatinib in a gastrointestinal stromal tumor patient. Gastroenterology. 2004. 127:294–299.

10. Chen LL, Sabripour M, Andtbacka RH, Petel SR, Feig BW, Macapinlac HA, et al. Imatinib resistance in gastrointestinal stromal tumors. Curr Oncol Rep. 2005. 7:293–299.

11. Debiec-Rychter M, Cools J, Dumez H, Sciot R, Stul M, Mentens N, et al. Mechanisms of resistance to imatinib mesylate in gastrointestinal stromal tumors and activity of the PKC412 inhibitor against imatinib-resistant mutants. Gastroenterology. 2005. 128:270–279.

12. Nilsson B, Bümming P, Meis-Kindblom JM, Odén A, Dortok A, Gustavsson B, et al. Gastrointestinal stromal tumors: the incidence, prevalence, clinical course, and prognostication in the preimatinib mesylate era--a population-based study in western Sweden. Cancer. 2005. 103:821–829.

13. Tryggvason G, Gislason HG, Magnússon MK, Jónasson JG. Gastrointestinal stromal tumors in Iceland, 1990-2003: the icelandic GIST study, a population-based incidence and pathologic risk stratification study. Int J Cancer. 2005. 117:289–293.

14. Morabito A, De Maio E, Di Maio M, Normanno N, Perrone F. Tyrosine kinase inhibitors of vascular endothelial growth factor receptors in clinical trials: current status and future directions. Oncologist. 2006. 11:753–764.

15. Demetri GD, von Mehren M, Blanke CD, Van den Abbeele AD, Eisenberg B, Roberts PJ, et al. Efficacy and Safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002. 347:472–480.

16. Haller F, Detken S, Schulten HJ, Happel N, Gunawan B, Kuhlgatz J, et al. Surgical management after neoadjuvant imatinib therapy in gastrointestinal stromal tumours (GISTs) with respect to imatinib resistance caused by secondary KIT mutations. Ann Surg Oncol. 2007. 14:526–532.

17. DeMatteo RP, Lewis JJ, Leung D, Mudan SS, Woodruff JM, Brennan MF. Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg. 2000. 231:51–58.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download