Abstract
Purpose
The serum concentrations of insulin-like growth factors-I (IGF-I), insulin-like growth factor binding protein-3 (IGFBP-3) and growth hormone (GH) are related to body composition, function and metabolism, and are influenced by the aging process. This study was to investigate the influence of gender on serum concentrations of IGF-I, IGFBP-3 and GH in middle and old age subjects.
Materials and Methods
Sixty healthy volunteers (male 35, female 25, 36-70 years) were divided into ≤ 50 and > 50 years groups, based on gender. Women > 50 years were post-menopause. IGF-I, IGFBP-3, and GH were determined by immunoradiometric assay.
Results
IGF-I was shown to be negatively correlated with age (women r = -0.62, p < 0.001; men r = -0.38, p < 0.05), whereas there was no correlation between IGF-I and GH values. Women > 50 years showed a significant reduction in IGF-I values than women ≤ 50 years (p < 0.01). Women > 50 years showed smaller IGF-I/IGFBP-3 molar ratios (0.177998 ± 0.039404) than men of same age group (0.228326 ± 0.050979, p < 0.01) and women ≤ 50 years (0.247667 ± 0.069411, p < 0.01). Age was shown to positively correlate with GH/IGF-I (r = 0.49, p < 0.05) and GH/IGFBP-3 ratios (r = 0.40, p < 0.05) in women.
The insulin-like growth factors (IGFs) are 7-kDa polypeptides that share structural homology with proinsulin.1 These proteins have growth-promoting properties in numerous tissues and are unable to suppress their bioactivity with anti-insulin antibodies.1,2 Both IGF-I and II are mitogens and IGF-II is much more active during prenatal life than IGF-I.3 IGF-I may be related to cognitive performance4-8 and neoplasia,3,9-23 and acts as a peripheral neuroactive signal participating not only in protection against injury, but also in normal brain function. Declining levels of serum IGF-I during aging may contribute to age-associated cognitive loss. IGF-I may promote cell cycle progression and inhibition of apoptosis either by directly associating with other growth factors or indirectly by interacting with other molecular systems which play an established role in carcinogenesis and cancer promotion, such as steroid hormones and integrins.15 Experimental and clinical studies suggest that increased serum levels of IGF-I and/or altered levels of their binding proteins are associated with increased risk of developing several malignancies.9-12,14-23 However, patients with hepato-cellular carcinoma were found to have lower IGF-I levels than those without hepatocellular carcinoma.13 Inap-propriate expression of the GH-IGF-I axis may contribute to cancer development.19 In extracellular tissues, circulating IGF-I is carried by a family of insulin-like growth factor binding proteins: more than 75% of circulating IGF-I is carried in a trimeric complex composed of IGF binding protein-3 (IGFBP-3), the main IGFBP in the serum, and a liver-derived glycoprotein known as the acid-labile subunit.24 IGF-I and IGFBPs are produced in several tissues, however, are mainly synthesized in the liver.25,26 IGF-I, IGFBP-3, and acid-labile subunit can be induced by human growth hor-mone (GH) and are affected by states of excess or deficiency of GH.27 Besides GH, circulating IGF-I concentrations can also be affected by many factors.3 Aging is an indirect deter-minant to affect the circulating IGF-I concentrations through its influence on GH-IGF-I axis.3 The activity of the GH-IGF-I axis undergoes an age-related reduction, and both spontaneous GH secretions and IGF-I values in the elderly subjects are frequently low.28-31 Estrogens and androgens are not only the direct determinants to affect the circulating IGF-I concentrations, but also indirect determinants through their influence on GH-IGF-I axis.3 Serum concentrations of estrogens in postmenopausal women are quite different from women with menstruation. Serum concentrations of androgens in men of over 50 age years may be different from men with age less than 50 years.32-36 Therefore, middle aged adults will undergo remarkable changes in their sex hormones which may have the possibility to influence the serum concentrations of IGF-I. The serum concentrations of IGFBP-3 and GH may also be changed with IGF-I variation. Age-related changes in body composition, function and metabolism may be related to changes in serum concentrations of IGF-I, IGFBP-3, and GH.28,29 Women are known to have an increased incidence of age-related diseases such as osteoporosis, Alzhelmer's disease, urinary incontinence, and coronary atherosclerosis after menopause.37 Although changes in female sex hormones may be the main reason,37 changes in serum IGF-I, IGFBP-3, and GH concentrations may also play some roles.3,28,29 Rapid increases in the incidence of age-related diseases in women is not observed in men. This could be due to the fact that sex hormonal changes proceed gradually in men compared to the sudden and fast changes that occur in women.37 Therefore, the patterns of changes in serum IGF-I, IGFBP-3, and GH concentrations in men may be quite different from women. However, the influence of gender on serum concentrations of IGF-I, IGFBP-3, and GH in middle and old aged subjects has not yet been well studied. The purpose of this prospective study was to clarify this issue. Our results may contribute to understanding the influence of gender on the aging process.
A total of 60 healthy volunteers (male 35, female 25, age range 36-70 years) were divided into ≤ 50 years and > 50 years groups, based on the gender (Table 1). All female subjects were questioned for their menstrual status at the start of the study. Female subjects without menstruation for at least one year were defined as post-menopausal. Female subjects > 50 years with menstrual cycle or ≤ 50 years without menstrual cycle and subjects with surgical history in gynecologic field were not included. None of the female volunteers had been treated by any hormone therapy. All female volunteers with age more than 50 years were post-menopause. None of the volunteers had history of cardiac disease, hypertension, pulmonary disease, diabetes mellitus, renal disease, or liver disease. All volunteers received health examinations. The renal and hepatic function, blood cell counts, alpha-fetoprotein, tissue polypeptide antigen, carcinoembryonic antigen, carbohydrate antigen 19-9, carbohydrate antigen 125, carbohydrate antigen 153, and thyroid stimulating hormones were all within their normal limits. None of the volunteers was positive for hepatitis B surface antigen or hepatitis C antibody. This study was approved by the Institutional Review Board of Kaohsiung Medical University Hospital (ClinicalTrials.gov NCT0067 9692). Each volunteer gave informed consent to participate in this study.
All volunteers fasted overnight for blood sampling. The serum used for the study was collected by centrifugation (3,500 g, 20 minutes) of blood immediately drawn from the volunteer, and was stored at -20℃ for further investigation. Serum values of IGF-I, IGFBP-3, and GH were determined by a solid phase two-site immunoradiometric assay using DSL-2800 ACTIVE® Non-Extraction IGF-I IRMA Kit, DSL-6600 ACTIVE® IGFBP-3 IRMA Kit (Diagnostic Systems Laboratories Inc, Webster, TX, USA), and CIS bio international hGH-RIACT kit (CIS bio international, Bagnols sur Cèze, France), respectively. The detection limit for IGF-I by this method was 2.06 ng/mL, and there was no cross-reaction with IGF-II, GH and insulin. The IGFBP-3 determined by our method was the total amount of IGFBP-3 which is the sum of intact and fragmented IGFBP-3. The detection limit for IGFBP-3 by this method was 0.5 ng/mL, and there was no cross-reaction with IGF-I, IGFBP-2 and IGFBP-5. The detection limit for the GH was 0.03 µIU/mL. The whole procedures for IGF-I, IGFBP-3 and GH determinations were performed by a same person to avoid inter-operator bias. The coefficient of variation values of intra-assay for the operator were 6.2%, 0.4% and 0.4% at IGF-I mean values (calculation from three determinations) of 34.01, 341.66 and 1181.10 ng/mL; 2.0%, 1.3% and 3.7% at IGFBP-3 mean values of 404.67, 2235.3 and 4511.3 ng/mL; and 0.3%, 1.6% and 0.5% at GH mean values of 0.26, 14.54 and 67.29 µIU/mL, respectively. The coefficient of variation values of inter-assay for the kits were 2.8%, 5.5% and 1.1% at IGF-I mean values (calculation from 3 determinations of 1 sample by each kit) of 35.76, 362.72 and 1189.6 ng/mL, 1.1%, 1.9% and 0.5% at IGFBP-3 mean values of 406.0, 2212.6 and 4398.0 ng/mL; and 3.2%, 2.2% and 4.4% at GH mean values of 0.28, 14.97 and 71.29 µIU/mL, respectively.
Data were expressed as mean ± standard deviation (SD). The 2-tailed t-test was used to analyze the significance of any difference between 2 means. Correlation analysis was investigated by the calculation of a correlation coefficient (r). Statistical significance was defined as p < 0.05.
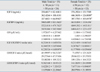
IGF-I showed a significantly negative correlation with age in 1) total volunteers (r = -0.51, p < 0.0001), 2) male volunteers (r = -0.38, p < 0.05), and 3) female volunteers (r = -0.62, p < 0.001). There was no difference in IGF-I values between male and female volunteers, and between male volunteers with ages ≤ 50 years and > 50 years. Female volunteers with ages > 50 years showed a significant reduction in IGF-I values than female volunteers with ages ≤ 50 years (p < 0.01) (Table 2).
IGFBP-3 did not show correlation with age in total, male and female volunteers. Female volunteers showed significantly higher IGFBP-3 values than male volunteers. There was no significant difference in IGFBP-3 values between male or female volunteers with ages ≤ 50 years and > 50 years (Table 2).
The variation of GH values was very large in male and female volunteers. GH did not show correlation with ages in total, male and female volunteers. There was no difference in GH values between the male and female volunteers, and between male or female volunteers with ages ≤ 50 years and > 50 years (Table 2).
There was a significantly positive correlation between IGF-I and IGFBP-3 values in 1) total volunteers (r = 0.46, p < 0.001), 2) volunteers with ages ≤ 50 years (r = 0.46, p < 0.05), 3) volunteers with ages > 50 years (r = 0.41, p < 0.05), 4) total female volunteers (r = 0.52, p < 0.01), and 5) female volunteers with ages > 50 years (r = 0.64, p < 0.05). Male volunteers and female volunteers with ages < 50 years did not show correlation between IGF-I and IGFBP-3 values. IGF-I/IGFBP-3 molar ratio showed a significantly negative correlation with age in 1) total volunteers (r = -0.35, p < 0.01), and 2) female volunteers (r = -0.64, p < 0.001). Male volunteers did not show correlation between IGF-I/IGFBP-3 molar ratio and age. Women > 50 years showed a smaller IGF-I/IGFBP-3 molar ratio (0.177998 ± 0.039404) than men of same age group (0.228326 ± 0.050979, p < 0.01) and women < 50 years (0.247667 ± 0.069411, p < 0.01) (Table 2).
There was no correlation between GH and IGF-I values in 1) total volunteers, 2) total male or female volunteers, 3) male or female volunteers with ages ± 50 years, and 4) male or female volunteers with ages > 50 years. GH/IGF-I ratio (µIU/nmol) showed a significantly positive correlation with age in 1) total volunteers (r = 0.27, p < 0.05), and 2) female volunteers (r = 0.49, p < 0.05). There was no difference in GH/IGF-I ratio (µIU/nmol) between 1) total male and female volunteers, 2) male and female volunteers with ages < 50 years, 3) male and female volunteers with ages > 50 years, and 4) male or female volunteers with ages ≤ 50 years and > 50 years (Table 2).
There was a significantly positive correlation between GH and IGFBP-3 values in 1) total male volunteers (r = 0.39, p < 0.05), and 2) male volunteers with ages > 50 years (r = 0.42, p < 0.05). GH/IGFBP-3 ratio (µIU/nmol) showed a significantly positive correlation with age in female volunteers (r = 0.40, p < 0.05). There was no difference in GH/IGFBP-3 ratio (µIU/nmol) between 1) total male and female volunteers, 2) male and female volunteers with ages ≤ 50 years, 3) male and female volunteers with ages > 50 years, and 4) male or female volunteers with ages ≤ 50 years and > 50 years (Table 2).
There was no correlation between GH values and IGF-I/IGFBP-3 molar ratios in 1) total volunteers, 2) total male and female volunteers, 3) male or female volunteers with ages ≤ 50 years, and 4) male or female volunteers with ages > 50 years. The ratios of GH values to IGF-1/IGFBP-3 molar ratios did not show correlation with age in total, male and female volunteers.
Circulating IGF-I concentrations can be affected through direct determinants such as protein-calorie intake, catabolic stressors, thyroxine, insulin, binding affinity of acid-labile subunit for IGF-I/IGFBP-3, zinc, parathyroid hormone, parathyroid hormone-related peptide, and platelet-derived growth factor.3 Estrogens, androgens, adrenal androgens and inflammatory cytokines are not only the direct determinants to affect the circulating IGF-I concentrations, but also indirect determinants through their influence on GH-IGF-I axis.3 Aging, body fat, and exercise are indirect determinants to affect circulating IGF-I concentrations through GH-IGF-I axis.3 Serum IGF-I concentrations can be measured by different immunoassays.38 The data obtained by our method are higher than those by other immunoassays (RIA-NICHOLS, ICMA-IMMULITE and IRMA-IMMUNOTECH methods).38 However, the difference in methodology will not influence the clinical application of serum IGF-I measurement.38 Comparison of our results with other studies should consider this difference. In the present study, serum IGF-I values showed a significantly negative correlation with age. This correlation was more prominent in female volunteers than in male volunteers. However, there was no significant difference in serum IGF-I values between male and female volunteers. This result was in accordance with one previous study,38 but different from the study with elderly subjects (mean age 71.6 years) in which serum IGF-I was inversely correlated with age only in men, and men had higher IGF-I values than women.39 The discrepancy may be due to small sample size in the present study or difference in subjects studied. Our subjects included both women with and without menstruation. Serum IGF-I values will significantly reduce in post-menopausal women as shown in our results. Estradiol is known to stimulate IGF-I synthesis and secretion from nonhepatic tissue.29 Decrease of estradiol in post-menopausal women may be responsible for the decrease of IGF-I in this population. GH is known to be an important factor to influence the circulating IGF-I concentrations. However, GH did not show a significant correlation with age in both male and female volunteers. This result was in accordance with the previous study using healthy subjects (30-60 years).40 The releasable pool of GH is generally preserved during the human life span.41 This may be the explanation for the lack of correlation between GH values and age in our study. No significant correction between serum IGF-I and GH values in the present study indicates that GH is not an important determinant for the serum IGF-I values in healthy middle and old age subjects. Aging with prominent changes in sex hormones caused by menopause may be the main determinant for the regulation of IGF-I in middle and old age women. In contrast to female volunteers, the factor of age over 50 years in male volunteers did not show significant influence on IGF-I levels. The fact that changes of androgens in men are not so clear-cut like menopause in female may be the explanation.37
The GH/IGF-I and GH/IGFBP-3 ratios represent how much concentrations of GH are required to maintain the serum concentrations of IGF-I and IGFBP-3. Increase of GH/IGF-I and GH/IGFBP-3 ratios indicates that the sensitivity of IGF-I and IGFBP-3 production to GH stimulation decreases. Although there was a positive correlation between GH/IGF-I ratio and age in all volunteers, this correlation was observed in the female volunteers, but the degree of this correlation was weak, indicating the trend of progressive hypoactivity of GH to stimulate IGF-I secretion with age in middle and old age women, but not in men. Estrogen can regulate not only GH secretion at the hypo-thalamic and pituitary level, but also can affect GH action at the level of the GH receptor expression and function.42 Estrogen has been shown to inhibit Janus kinase/signal transducer and activator of transcription signaling by GH via the induction of suppressor of cytokine signaling-2.42 Estrogen provides one explanation for the relative GH resistance observed in women.42,43 Women are in a state of persistent estrogen-modulated GH resistance for a long time43 which may cause progressive hypoactivity of GH to stimulate IGF-I secretion with age. Although the correlation was weak, GH/IGFBP-3 ratio also increased with age, similar to GH/IGF-I ratio with age in female volunteers. Since both IGF-I and insulin are necessary for maintenance of normal IGFBP-3 production and IGF-I directly regulates synthesis of IGFBP-3,44 increase of GH/IGFBP-3 ratio with age may be via hypoactivity of GH to stimulate IGF-I secretion.
The bioavailability of IGF-I to the tissues is influenced by serum IGFBP concentrations as well as IGFBP proteolytic activity. The IGF-I/IGFBP-3 molar ratio can reflect theoretically free, biologically active IGF-I.29 Decreases in this ratio suggests reduction of IGF-I activity.45,46 Although the present study showed that female volunteers had significantly higher IGFBP-3 values than male volunteers, there was no significant difference in IGF-I/IGFBP-3 molar ratio between male and female volunteers with age ≤ 50 years. Since there was no correlation between IGFBP-3 and age, significant decreases of IFG-I in post-menopausal women caused significant reduction of IGF-I/IGFBP-3 molar ratio in this group. Post-menopausal women have reduced biologically active IGF-I. On the other hand, only women volunteers showed a positive correlation between serum IGF-I and IGFBP-3 values. Although female volunteers with ages ≤ 50 years did not show this correlation, small sample size may be the explanation. However, there was a significant correlation between serum IGF-I and IGFBP-3 values in post-menopausal women, even though the sample size was small. This indicates that the correlated secretions between IGF-I and IGFBP-3 become more obvious in post-menopausal women than women with menstruation.
Only male volunteers showed a significantly positive correlation between GH and IGFBP-3 values. However, this correlation was actually observed in those with ages > 50 years. This suggests that IGFBP-3 secretion is significantly influenced by GH status only in men over 50 years.
No correlation between GH values and IGF-I/IGFBP-3 molar ratios indicates that GH concentrations in healthy middle and old age subjects are not significantly influenced by the changes in free IGF-I concentrations. This phenomenon will persist during middle and older because the ratios of GH values to IGF-I/IGFBP-3 molar ratios also did not show correlation with age in the present study.
The present study did not concomitantly determine the serum sex hormones values. This makes us unable to evaluate the actual impact of sex hormones on IGF-I, IGFBP-3 and GH production. All blood sampling in female before menopause were random without selection in any particular menstrual phase. Serum IGF-I levels fluctuate with estrogen levels during menstrual cycle, however, this fluctuation in serum IGF-I levels is usually mild.43
In conclusion, IGF-I progressively decrease with age, which is not correlated with GH. Influence of aging on serum concentrations of IGF-I is more remarkable in women than in men. Menopause causes reduction of IGF-I/IGFBP-3 molar ratio. Women have the trend of progressive hypoactivity of GH to stimulate IGF-I and IGFBP-3 secretions with age.
Figures and Tables
References
1. Zapf J, Schmid C, Froesch ER. Biological and immunological properties of insulin-like growth factors (IGF) I and II. Clin Endocrinol Metab. 1984. 13:3–30.
2. Jones JI, Clemmons DR. Insulin-like growth factors and their binding proteins: biological actions. Endocr Rev. 1995. 16:3–34.
3. Rosen CJ. Serum insulin-like growth factors and insulin-like growth factor-binding proteins: clinical implications. Clin Chem. 1999. 45:1384–1390.

4. van Dam PS, Aleman A. Insulin-like growth factor-I, cognition and brain aging. Eur J Pharmacol. 2004. 490:87–95.

5. Sonntag WE, Ramsey M, Carter CS. Growth hormone and insulin-like growth factor-1 (IGF-1) and their influence on cognitive aging. Ageing Res Rev. 2005. 4:195–212.

6. Aberg ND, Brywe KG, Isgaard J. Aspects of growth hormone and insulin-like growth factor-I related to neuroprotection, regeneration, and functional plasticity in the adult brain. ScientificWorldJournal. 2006. 6:53–80.

7. Fernandez S, Fernandez AM, Lopez-Lopez C, Torres-Aleman I. Emerging roles of insulin-like growth factor-I in the adult brain. Growth Horm IGF Res. 2007. 17:89–95.

8. Trejo JL, Piriz J, Llorens-Martin MV, Fernandez AM, Bolós M, LeRoith D, et al. Central actions of liver-derived insulin-like growth factor I underlying its pro-cognitive effects. Mol Psychiatry. 2007. 12:1118–1128.

9. Chan JM, Stampfer MJ, Giovannucci E, Gann PH, Ma J, Wilkinson P, et al. Plasma insulin-like growth factor-I and prostate cancer risk: a prospective study. Science. 1998. 279:563–566.
10. Hankinson SE, Willett WC, Colditz GA, Hunter DJ, Michaud DS, Deroo B, et al. Circulating concentrations of insulin-like growth factor-I and risk of breast cancer. Lancet. 1998. 351:1393–1396.

11. Cohen P. Serum insulin-like growth factor-I levels and prostate cancer risk--interpreting the evidence. J Natl Cancer Inst. 1998. 90:876–879.

12. Ma J, Pollak MN, Giovannucci E, Chan JM, Tao Y, Hennekens CH, et al. Prospective study of colorectal cancer risk in men and plasma levels of insulin-like growth factor (IGF)-I and IGF-binding protein-3. J Natl Cancer Inst. 1999. 91:620–625.

13. Stuver SO, Kuper H, Tzonou A, Lagiou P, Spanos E, Hsieh CC, et al. Insulin-like growth factor 1 in hepatocellular carcinoma and metastatic liver cancer in men. Int J Cancer. 2000. 87:118–121.
14. Yu H, Rohan T. Role of the insulin-like growth factor family in cancer development and progression. J Natl Cancer Inst. 2000. 92:1472–1489.

15. Moschos SJ, Mantzoros CS. The role of the IGF system in cancer: from basic to clinical studies and clinical applications. Oncology. 2002. 63:317–332.

16. Mazziotti G, Sorvillo F, Morisco F, Carbone A, Rotondi M, Stornaiuolo G, et al. Serum insulin-like growth factor I evaluation as a useful tool for predicting the risk of developing hepatocellular carcinoma in patients with hepatitis C virus-related cirrhosis: a prospective study. Cancer. 2002. 95:2539–2545.

17. Mattera D, Capuano G, Colao A, Pivonello R, Manguso F, Puzziello A, et al. Increased IGF-I: IGFBP-3 ratio in patients with hepatocellular carcinoma. Clin Endocrinol (Oxf). 2003. 59:699–706.
18. Ibrahim YH, Yee D. Insulin-like growth factor-I and cancer risk. Growth Horm IGF Res. 2004. 14:261–269.

19. Jenkins PJ, Bustin SA. Evidence for a link between IGF-I and cancer. Eur J Endocrinol. 2004. 151:Suppl 1. S17–S22.

20. Elsammak MY, Amin GM, Khalil GM, Ragab WS, Abaza MM. Possible contribution of serum activin A and IGF-1 in the development of hepatocellular carcinoma in Egyptian patients suffering from combined hepatitis C virus infection and hepatic schistosomiasis. Clin Biochem. 2006. 39:623–629.

21. Peeters PH, Lukanova A, Allen N, Berrino F, Key T, Dossus L, et al. Serum IGF-I, its major binding protein (IGFBP-3) and epithelial ovarian cancer risk: the European Prospective Investigation into Cancer and Nutrition (EPIC). Endocr Relat Cancer. 2007. 14:81–90.

22. Baglietto L, English DR, Hopper JL, Morris HA, Tilley WD, Giles GG. Circulating insulin-like growth factor-I and binding protein-3 and the risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 2007. 16:763–768.

23. Serin IS, Tanriverdi F, Yilmaz MO, Ozcelik B, Unluhizarci K. Serum insulin-like growth factor (IGF)-I, IGF binding protein (IGFBP)-3, leptin concentrations and insulin resistance in benign and malignant epithelial ovarian tumors in postmenopausal women. Gynecol Endocrinol. 2008. 24:117–121.

24. Holman SR, Baxter RC. Insulin-like growth factor binding protein-3: factors affecting binary and ternary complex formation. Growth Regul. 1996. 6:42–47.
25. Daughaday WH, Rotwein P. Insulin-like growth factors I and II. Peptide, messenger ribonucleic acid and gene structures, serum, and tissue concentrations. Endocr Rev. 1989. 10:68–91.

26. Arany E, Afford S, Strain AJ, Winwood PJ, Arthur MJ, Hill DJ. Differential cellular synthesis of insulin-like growth factor binding protein-1 (IGFBP-1) and IGFBP-3 within human liver. J Clin Endocrinol Metab. 1994. 79:1871–1876.

27. Blum WF, Albertsson-Wikland K, Rosberg S, Ranke MB. Serum levels of insulin-like growth factor I (IGF-I) and IGF binding protein 3 reflect spontaneous growth hormone secretion. J Clin Endocrinol Metab. 1993. 76:1610–1616.

28. Ghigo E, Arvat E, Gianotti L, Ramunni J, DiVito L, Maccagno B, et al. Human aging and the GH-IGF-I axis. J Pediatr Endocrinol Metab. 1996. 9:Suppl 3. 271–278.
29. Arvat E, Broglio F, Ghigo E. Insulin-Like growth factor I: implications in aging. Drugs Aging. 2000. 16:29–40.
30. Lombardi G, Di Somma C, Rota F, Colao A. Associated hormonal decline in aging: is there a role for GH therapy in aging men? J Endocrinol Invest. 2005. 28(3):Suppl. 99–108.
31. Sherlock M, Toogood AA. Aging and the growth hormone/insulin like growth factor-I axis. Pituitary. 2007. 10:189–203.

33. Cuzin B, Giuliano F, Jamin C, Legros JJ, Lejeune H, Rigot JM, et al. [Investigation, treatment and surveillance of late-onset hypogonadism in males: the official guidelines of the International Society for the Study of the Aging Male (ISSAM) with comments]. Prog Urol. 2004. 14:1–14.
34. Castro-Acuna V, Martínez-Martínez L, Larrea F. [Partial androgen deficiency in the aging male]. Rev Invest Clin. 2004. 56:507–512.
35. Lenk VS. [Diagnosis of the "aging male"--what is recommended?]. Urologe A. 2005. 44:1167–1172.
37. Jung BH, Jeon MJ, Bai SW. Hormone-dependent aging problems in women. Yonsei Med J. 2008. 49:345–351.

38. Granada ML, Ulied A, Casanueva FF, Pico A, Lucas T, Torres E, et al. Serum IGF-I measured by four different immunoassays in patients with adult GH deficiency or acromegaly and in a control population. Clin Endocrinol (Oxf). 2008. 68:942–950.

39. Kiel DP, Puhl J, Rosen CJ, Berg K, Murphy JB, MacLean DB. Lack of an association between insulin-like growth factor-I and body composition, muscle strength, physical performance or self-reported mobility among older persons with functional limitations. J Am Geriatr Soc. 1998. 46:822–828.

40. Vahl N, Jørgensen JO, Skjaerbaek C, Veldhuis JD, Orskov H, Christiansen JS. Abdominal adiposity rather than age and sex predicts mass and regularity of GH secretion in healthy adults. Am J Physiol. 1997. 272:E1108–E1116.

41. Ghigo E, Arvat E, Gianotti L, Lanfranco F, Broglio F, Aimaretti G, et al. Hypothalamic growth hormone-insulin-like growth factor-I axis across the human life span. J Pediatr Endocrinol Metab. 2000. 13:Suppl 6. 1493–1502.

42. Leung KC, Johannsson G, Leong GM, Ho KK. Estrogen regulation of growth hormone action. Endocr Rev. 2004. 25:693–721.

43. Gleeson HK, Shalet SM. GH responsiveness varies during the menstrual cycle. Eur J Endocrinol. 2005. 153:775–779.

44. Binoux M. GH, IGFs, IGF-binding protein-3 and acid-labile subunit: what is the pecking order? Eur J Endocrinol. 1997. 137:605–609.

45. Juul A, Main K, Blum WF, Lindholm J, Ranke MB, Skakkebaek NE. The ratio between serum levels of insulin-like growth factor (IGF)-I and the IGF binding proteins (IGFBP-1, 2 and 3) decreases with age in healthy adults and is increased in acromegalic patients. Clin Endocrinol (Oxf). 1994. 41:85–93.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download