Abstract
Purpose
Chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS, NIH category III) accounts for 90-95% of prostatitis cases. However, standard treatment has not yet been established. It is known that polyphenols have an inhibitory effect on inflammation by their antioxidative capacity, and oligonol, a polyphenol derivative, has much higher bioavailability and bioactivity than common polyphenols. We investigated the anti-inflammatory effects and mechanisms of oligonol in estradiol-induced prostatitis rat models.
Materials and Methods
Prostatitis was induced by 17 beta-estradiol (E2) and dihydrotestosterone (DHT) in Wistar male rats (n = 20). Ten rats were placed in the oligonol-treated group and 10 in the E2 + DHT-treated group. The other 10 rats were also included as normal control group. Oligonol (60 mg/kg/day) was administered via gavage tube for 4 weeks. Superoxide dismutase (SOD), glutathione peroxidase (GPx), and tumor necrosis factor-alpha (TNF-α) were quantified, and phosphorylation of IκBa and histological changes were also evaluated in prostatic tissue.
Results
The SOD and GPx activity showed tendencies to increase in the oligonol-treated group compared to the normal control group. TNF-α expression was slightly reduced in the oligonol-treated group. Western blotting demonstrated that phosphorylation of IκBa in the oligonol-treated group was significantly lower than in the normal control group. The E2 + DHT-treated group revealed severe atrophy of acinar epithelial cells and infiltration of leukocytes and lymphocytes in the prostate, however, the oligonol-treated group showed overall reduction in inflammatory features.
Prostatitis is the most common urological diagnosis in men younger than 50 years and the third most common urologic diagnosis in men older than 50 years. An estimated 50% of all men experience prostatitis-like symptoms at some point during their lifetime.1 According to the National Institutes Health (NIH)/National Institute of Diabetes Digestire and Kidney Disease (NIDDK) classification of prostatitis in 1999, chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS) is the most prevalent type, accounting for at least 90% of prostatitis cases. The impact of CP on health status is significant and the quality of life of many patients with CP/CPPS is impaired. Because the etiology and pathogenesis of nonbacterial prostatitis remains unclear, however, it is difficult to treat nonbacterial prostatitis, especially when it becomes chronic.
Polyphenol, a secondary metabolite of plants, inhibits inflammation by activating various antioxidant enzymes with its hydroxyl group (-OH). Catechin in green tea, chlorogenic acid in coffee, and resveratrol in grapes are polyphenolic antioxidant plant metabolites. Polyphenol has a crucial role in prevention of degenerative diseases such as cancer and coronary heart disease.2,3 Green tea polyphenol effectively inhibits the production of interleukin (IL)-8, one of the most powerful chemoattractants for neutrophils in inflammatory A549 bronchial epithelial cells induced by IL-1β.4 Shoskes et al.5 reported that 67% of patients with category III CP taking the bioflavonoid quercetin had at least 25% improvement in symptom score.
Oligonol (Amino Up Chemical Co., Sapporo, Japan), an oligomerized polyphenol, is derived from depolymerization of catechin-type polyphenol and contains a large quantity of lower oligomers such as monomer, dimer, trimer, and tetramer: oligonol contains 50% of oligomers whereas a typical polyphenol polymer contains less than 10%. Thus, polyphenol polymers are not as efficiently bioactive or easily absorbed as oligonol because of high molecular weights. Fujii et al.6 demonstrated that oligonol showed better bioavailability than grape seed polyphenol and that oligonol is safe after repeated intake of doses lower than 200 mg/day.
Lewis and Wistar rats develop a spontaneous nonbacterial prostatitis with advancing age, making them good animal models for laboratory investigation of this disease.7 Administration of exogenous estradiol (E2) increases the incidence and severity of prostatitis in adult Wistar rats.7,8 According to Naslund et al.,7 spontaneously developed prostatitis and E2-induced prostatitis in Wistar rats had the same histologic findings. Furthermore, a number of studies demonstrated that spontaneous nonbacterial prostatitis in rats was histologically very similar to CP in humans.9,10
Nuclear factor kappa B (NF-κB) is expressed in all cells and is involved in both initiation and termination of inflammation. IκB is an inhibitor of NF-κB gene expression and restricts inflammation and growth of cancer. When IL-1 or tumor necrosis factor-alpha (TNF-α) binds their receptor, IκB kinase (IKK) induces phosphorylation and ubiquitination of IκB and NF-κB is separated from IκB and activated through entering the nucleus. The NF-κB pathway is inhibited by epigallocatechin-3-gallate, a green teaderived polyphenol, in IL-1β-treated A549 cells.11 In addition, resveratrol reduces lipopolysaccharide (LPS)-induced airway neutrophilia and inhibits activation of NF-κB and protein-1 (AP-1) in U937 and A549 cells.12-14 The role of NF-κB pathway has been shown in prostate cancer, but not in prostatitis. Therefore, more efforts are needed to investigate the mechanism of prostatitis development in relation to NF-κB which is activated by oxidative stress. The aim of this study was to demonstrate the anti-inflammatory and preventive effects of oligonol on the development of nonbacterial prostatitis in rat model, induced by estrogen.
Oligonol was supplied by Amino Up Chemical Co. (Sapporo, Japan), and produced by oligomerization of polyphenols rich in grape seeds. E2, testosterone (T), dihydrotestosterone (DHT), hematoxylin and eosin were purchased from Sigama-Aldrich (Steinheim, Germany). Superoxide dismutase (SOD) Assay Kit-WST was purchased from Dojindo (Gaithersburg, MD, USA). GPx Assay Kit was purchased from Cayman Chemical (Ann Arbor, MI, USA). TNF-α immunoassay kit was purchased from PEPROTECH (Rocky Hill, NJ, USA). Anti-IKBa, anti-pIKBa, and β-actin antibodies were purchased from Cell Signaling Technology (Danvers, MA, USA).
Adult Wistar male rats (3 month old) were purchased from Central Lab Animal Inc. (Seoul, Korea). Rats were housed in an animal room maintained at constant temperature and humidity with a 12-hour light/dark cycle. The treatment protocols were approved by the Institutional Animal Care and Use Committee at Yonsei University. We used the modified model of Robinette15 and Naslund7 with estrogen treatment of Wistar male rats to induce inflammation in the lateral prostatic lobe. Rats were divided into 3 groups of 10 rats each: (I) the normal control group, (II) E2 + DHT-treated group, and (III) oligonol-treated group. The experimental protocol is shown in Table 1 and the schedule is illustrated in Fig. 1. E2 and DHT were dissolved in sesame oil and injected subcutaneously at a dose of 250 µg/kg/day. Oligonol was diluted in drinking water. In the normal control group, rats were treated only with sesame oil, but without drugs. In the E2 + DHT-treated group, E2 was injected subcutaneously into the back of rats for 4 weeks and DHT was added for 2 weeks from day 15. Rats in the oligonol-treated group received oral administration of 60 mg/kg of oligonol for 4 weeks and E2 and DHT were injected in the same manner as the E2 + DHT-treated group.
Rats were weighed every 7 days (day 1, 8, 15, 22, and 29) and sacrificed on day 29, and the prostate was extirpated. After the prostate gland weight was measured without bladder or seminal vesicles, both lateral lobes of prostates were dissected and used for histopathologic evaluation. Thereafter, samples were fixed in neutral 10% formalin solution for 18 hours at room temperature, dehydrated in ethanol, cleared in xylene, and embedded in paraffin. For routine histological analysis with hematoxylin and eosin, the prostate lateral lobe sections of paraffin-embedded tissues were cut at 6 µm thickness. Four micron sections were taken from each lateral lobe of prostate and stained with hematoxylin and eosin. Tissues were considered positive for prostatitis if one area of inflammatory cell infiltration was observed in a microscopic section. The severity of inflammation was assessed according to the aggressiveness of inflammation and by counting the number of inflamed acini. Agressiveness of inflammation in the lateral lobe was determined according to the method applied for quantification of prostatic inflammation in the adult Noble rats by Bernoulli et al, judged on a grade of 0 to 3.16,17 Grade 0 meant no contact between inflammatory cells and epithelium; grade 1 some contact; grade 2 periglandular infiltrates adjacent to partially destroyed epithelium; and grade 3 the amount of these acini was more than 25%. The number of inflamed acini was counted for the whole lateral prostate area by using the same sections.
Blood samples were collected by heart puncture before neck dislocation. SOD is the enzyme that catalyzes the dismutation of superoxide (O-2-) into oxygen and hydrogen peroxide (H2O2), and plays a critical role in the defense of cells against the toxic effects of oxygen radicals. Total SOD activity (CuZnSOD and Mn SOD) in serum and lateral lobe of prostate tissue was measured by SOD Assay Kit-WST (Dojindo), monitoring the decrease in the rate of superoxide-mediated reduction of nitroblue tetrazolium at 450 nm using spectrophotometer. GPx catalyzes the reduction of hydroperoxides, including hydrogen peroxide, by reduced glutathione and functions to protect the cells from oxidative damage. Total GPx activity in plasma and lateral lobe of prostate tissue was measured by a GPx Assay Kit (Cayman), using spectrophotometer at 340 nm.
Blood obtained before sacrifice was centrifuged for 10 minutes (3,000 rpm, 4℃), and the supernatant was immediately transferred to a tube. TNF-α concentration was measured by TNF-α immunoassay kit (PEPROTECH) according to the manufacturer's protocol every 5 minutes for 30 minutes, using spectrophotometer at 450 nm.
To isolate protein, lateral prostates were washed with cold Phosphate befferd saline (PBS) and homogenized on ice. The tissues were solubilized with RIPA lysis buffer [50 mM HEPES (USB, Cleveland, OH, USA) (pH 7.6), 150 mM NaCl (Sigma, St. Louis, MO, USA), 1% NP-40 (Amresco, Solon, OH, USA), 10 µL/mL, phenylmethylsulfonyl fluoride (Amresco), and 10µL/mL aprotinin (Sigma)], and cleared by centrifugation. Protein concentration was determined using Bio-Rad Laboratories DC protein assay kits (Hercules, CA, USA). Equal amounts of cell lysates were resolved by Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDSPAGE), transferred to nitrocellulose membranes, and incubated with anti-IκBa (1 : 1000), anti-pIκBa (1 : 1000), and β-actin (1 : 2000) antibodies. Protein signals were detected by chemiluminescence with ECL detection reagents (Amersham Biosciences, Piscataway, NJ, USA). Protein immunoblots were quantified by imaging densitometry with Image Gauge Software (Fuji Photo Film). Results were presented as mean ± standard deviation (SD) of the optical density ratio between the gray values of nonphosphorylated and phosphorylated immunoblot bands for pIκBa/ IκBa and β-actin.
The E2 + DHT and oligonol-treated groups showed significantly reduced body weight compared to the normal control group on day 29. However, no significant difference in body weight was noted between the E2 + DHT and oligonol-treated group (Fig. 2A). The E2 + DHT and oligonol-treated groups showed significantly reduced prostate weight compared to the normal control group, however, no significant difference in prostate weight was noted between the two group (Fig. 2B). Side effects such as lethargy and mortality were not observed in response to oligonol treatment.
In the normal control group, there was a normal appearance of the glandular epithelium and stroma with no obvious leukocyte infiltration into the lumina and stroma that refers to grade 0 in all rats. On the other hand, extensive infiltration of inflammatory cells in the lumina, mononuclear cells in the stroma of the gland, and epithelial degeneration were observed in the E2 + DHT-treated group, suggesting CP. Of the 10 rats in this group, 8 rats showed grade 3 and 2 showed grade 2 inflammation. In the oligonol-treated group, there were less inflammatory cells in the lumina and the epithelial cells of gland and stroma showed more improvement compared to the E2 + DHT-treated group. In the oligonol-treated group, 6 rats showed grade 2 and another 4 presented grade 1 inflammation (Fig. 3).
SOD activity was measured in serum and lateral lobes of prostate tissue of the sacrificed rats. In serum, SOD activity was 33.9 ± 7.05, 30.77 ± 3.7, and 32.93 ± 9.45 (units/mL) for the normal control, E2 + DHT-treated, and oligonol-treated groups, respectively. There were no significant differences in serum SOD activity among the 3 groups (Fig. 4A). In lateral lobe of prostate tissue, SOD activity was 37.82 ± 1.98, 31.59 ± 3.57, and 37.01 ± 3.95 (units/mL) for the normal control, E2 + DHT-treated, and oligonol-treated groups, respectively. The decrease in tissue SOD activity in the E2 + DHT-treated group was statistically significant (p < 0.05). However, there was no significant difference in tissue SOD activity between the E2 + DHT and oliginol-treated groups, even though increased tissue SOD activity was observed in the oligonol-treated group (Fig. 4B).
GPx activity was measured in plasma and lateral lobes of prostate tissue of sacrificed rats. In plasma, GPx activity was 13.58 ± 1.47, 6.37 ± 1.8, and 11.46 ± 1.8 (units/mL) for the normal control, E2 + DHT-treated, and oligonol-treated groups, respectively. In lateral lobe of prostate tissue, GPx activity was 358.94 ± 90.91, 173.19 ± 13.3, and 210.12 ± 67.25 (units/mL) for the normal control, E2 + DHT-treated, and oligonol-treated group, respectively. In both plasma and prostate tissue, GPx activity was significantly decreased in the E2 + DHT-treated group compared to the normal control group (p < 0.05). The oligonol-treated group showed significantly increased GPx activity in plasma and prostate tissue compared to the E2 + DHT-treated group (p < 0.05) (Fig. 5).
TNF-α, a pleotropic inflammatory cytokine, in rat serum was quantitatively determined. A significant 9.6-fold increase in TNF-α was detected when the E2 + DHT-treated group was compared to the normal control group (62.5 ± 10.61 and 6.5 ± 4.95 pg/mL, respectively; p < 0.05). There was also a decrease in TNF-α in the oligonol-treated group compared to the E2 + DHT-treated group (41.25 ± 17.46 and 62.5 ± 10.61 pg/mL, respectively), however, a significant difference was not noted (Fig. 6).
We determined the effects of E2 + DHT and oligonol on the activation of NF-κB by using IκBa phosphorylation. Analysis with Western blot showed that phosphorylation of IκBa was increased in the E2 + DHT-treated group compared to the normal control group (p < 0.05), and the oligonol-treated group showed decreased phosphoryltaion of IκBa compared to the E2 + DHT-treated group (p < 0.05) (Fig. 7). These results indicate that oligonol inhibits E2-induced CP by regulating phosphorylation of IκBa.
The etiology and pathogenesis of nonbacterial prostatitis remains unclear. Even though several treatment modalities have been applied, it is difficult to treat nonbacterial prostatitis, especially when it becomes chronic. We assumed that the application of oligonol would be helpful for treating nonbacterial CP by regulating antioxidatives, proinflammatory cytokines, and IKBa phosphorylation.
Estrogen-induced prostatitis is partly related to the inhibition of dopamine secretion at the hypothalamus, and dopamine deficiency enhances the production and secretion of prolactin that eventually causes inflammation of the prostate.18,19 Tangbanluekal et al. reported that rats with E2-induced prostatitis were correlated with increased serum prolactin, and elevated pituitary weight, and that the administration of bromocryptine, a dopamine D2 agonist, was effective in suppressing pituitary weight and hyperprolactinemia and mitigated the lateral prostate inflammation. They also showed that inflammation was restored in the bromocryptine-treated hormone-implanted rats by administering exogenous ovine prolactin. These results indicate that E2-induced inflammation in the rat lateral prostate is mediated at least in part by the release of prolactin from the pituitary.19 Administration of E2 for 2 weeks results in inflammation of the rat prostate. Injection of testosterone with E2 prevents atrophy of tissue as well as inflammation, but DHT maintains the inflammation induced by E2 and prevents atrophy of tissue.20 We utilized the modified model of Robinette and Naslund with E2 treatment for 4 weeks and DHT treatment for 2 weeks to induce inflammation in the lateral prostatic lobe in noncastrated, adult Wistar male rats. With this modified mothod, we observed chronic inflammation of the lateral lobe of rat prostate. The injected dose of E2 and DHT to induce inflammation was 250 µg/kg/day which is the same dose as in the Naslund's model.
Since the etiology of nonbacterial prostatitis has not yet been established, various factors have been reported, including Trichomonas vaginalis, Chlamydia trachomatis, genital mycoplasmas, Staphylococci, Coryneforms, genital viruses, biofilm, stagnation of prostatic secretion, autoimmune diseases, allergy, sex hormone disorders, and psychological factors.21-23 In the present study, oxidative stress was investigated as a cause of CP.
We applied the 4-point inflammation grading system, used by Bernoulli et al., in evaluating the severity of inflammation of lateral prostate lobes.16 The severity of inflammation was assessed according to the aggressiveness of inflammation and by counting the number of inflamed acini from grade 0 to grade 3. On microscopic findings, the oligonol-treated group showed an effectively reduced inflammation of the prostate and degeneration of the glandular epithelium compared to the E2 + DHT-treated group. E2 + DHT and oligonol-treated group showed significantly reduced prostate weight compared to the normal control group. The reduced prostate weight was most likely due to decreased secretion production. E2 given with DHT could restore wet prostate weight which does not include the secretion weight and could decrease secretion production as well.
The antioxidative effects of oligonol have been shown in several studies. Fujii et al.24 reported that oligonol treatment had potent protective effects against high glucose-induced oxidative stress and cytotoxicity in cultured LLC-PK1 cells, and Li et al.25 showed that oligonol attenuated β-amyloid-induced cytotoxicity, apoptotic features, intracellular reactive oxygen species (ROS) accumulation, and lipid peroxidation.
SOD converts superoxide into H2O2, and GPx stabilizes H2O2 to H2O and holds internal oxidation. SOD and GPx are well-known enzymes that prevent oxidation, and they can serve as an indicator of oligonol's antioxidative effects on CP in rats. We demonstrated that development of non-bacterial prostatitis significantly decreased SOD and GPx activity in prostate tissue. In particular, GPx activity was significantly increased in the oligonol-treated group, but SOD activity showed no statistical significance. Therefore, oligonol is expected to have an influence on GPx activation rather than SOD activation in the antioxidant process. According to studies on the relation between oxidative stress and prostatitis, decrease of catalase activity and increase of lipid peroxidation were observed in rats with autoimmune prostatitis.26 Additionally, decreased activity of anti-oxidative enzymes was reported in patients with chronic bacterial prostatitis.27
There have been efforts to calculate the scores of ROS and total antioxidant capacity (TAC) and to predict the possibility of infertility in patients with prostatitis. TAC levels and ROS-TAC scores in semen of patients with CP were much lower than in the control group, suggesting that antioxidant supplements might be helpful for patients with CP.28 ROS level was the highest in patients with CP as well as varicocele and ROS-TAC score was suggested as a possible predictor of infertility.29
In the present study, TNF-α, a proinflammatory cytokine, was significantly increased in rats with CP and was decreased in the oligonol-treated group without statistical significance. When TNF-α binds its receptor, IKK induces phosphorylation and ubiquitination of IκB and the NF-κB pathway is activated. Manna et al.13 reported that resveratrol blocked TNF-induced NF-κB activation in a dose- and time-dependent manner. Administration of 10 ng/mL TNF-α in A540 cells with oxidative stress of 100 µm hydrogen peroxide enhanced DNA binding of NF-κB, resulting in lung inflammation.30 Phosphorylated IKBa was significantly increased in rats with CP and was significantly decreased in the oligonol-administrated group, suggesting that oligonol inhibits E2-induced CP by regulating phosphorylation of Iκ Ba and the NF-κB pathway.
To our best knowledge, this study is the first attempt to investigate the effects of oligonol on chronic nonbacterial prostatitis with rat model. We demonstrated that oligonol improves estradiol-induced CP in rats by regulating antioxidatives, proinflammatory cytokine, phosphylation of IκBa, and the NF-κB pathway. Since E2-induced CP in rats is histolopathologically very similar to CP in humans, oligonol is expected to have beneficial effects on prevention and treatment of CP, especially CPPS IIIA in patients.
Figures and Tables
 | Fig. 2Effect of E2 +DHT and oligonol on body weight and prostate weight. (A) The E2 + DHT and oligonol-treated group showed significantly reduced body weight compared to the normal control group on day 29 (p < 0.001). No significant difference in body weight was noted between the E2 + DHT and oligonol-treated group (p = 0.952). (B) The E2 + DHT and oligonol-treated group showed significantly reduced prostate weight compared to the normal control group (p < 0.001). No significant difference in prostate weight was noted between the E2 + DHT and oligonol-treated group (p = 0.125). E2, 17 estradiol; DHT, dihydrotestosterone. |
 | Fig. 3Histopathologic findings of the prostate lateral lobe in 3 groups (hematoxylin and eosin stain, ×100). (A) The normal control group showed normal appearance of the glandular epithelium without leukocyte infiltration. All rats showed grade 0 inflammation. (B) In the E2 +DHT-treated group, extensive infiltration of inflammatory cells, including neutrophils, lymphocytes, macrophages, and degeneration of glandular epithelial cells, were found, suggesting CP. Eight rats showed grade 3 and 2 showed grade 2 inflammation. (C) In the oligonol-treated group, less inflammatory cells and degenerated epithelial cells were noted. Six rats showed grade 2 and 4 presented grade 1 inflammation. E2, 17 estradiol; DHT, dihydrotestosterone; CP, chronic prostatitis. |
 | Fig. 4Effect of E2 + DHT and oligonol on SOD activity in serum (A) and lateral lobe of prostate tissue (B). Each value represents mean ± SD. E2, 17 estradiol; DHT, dihydrotestosterone; SOD, superoxide dismutase. *Significantly different from the normal control group at p < 0.05. |
 | Fig. 5Effect of E2+DHT and oligonol on GPx activity in plasma (A) and lateral lobe of prostate tissue (B). Each value represents mean ± SD. E2, 17 estradiol; DHT, dihydrotestosterone. *Significantly different from the normal control group at p < 0.05. †Significantly different from the E2 + DHT-treated group at p < 0.05. |
 | Fig. 6Effect of E2 + DHT and oligonol on serum TNF-α level. Each value represents mean ± SD. E2, 17 estradiol; DHT, dihydrotestosterone; TNF-α, tumor necrosis factor-alpha. *Significantly different from the normal control group at p < 0.05. |
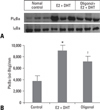 | Fig. 7Effect of E2 + DHT and oligonol on IκBa phosphorylation. A: pIκBa and Iκ Ba expression using Western blotting analysis. B: pIκBa immunoblots were quantified by imaging densitometry with Image Gauge Software. E2, 17 estradiol; DHT, dihydrotes-tosterone; Od, optical density; Bkg, background. *The E2 + DHTtreated group presented significant increase of pIκBa compared to the normal control group (p < 0.05). †The oligonol-treated group showed significant decrease of pIκBa compared to the E2 + DHT-treated group (p < 0.05). |
ACKNOWLEDGEMENTS
This study was supported by Brain Korea 21 Project for Medical Science and a faculty research grant from Yonsei University College of Medicine.
References
1. Fowler JE Jr. Gillenwater JA, Grayhack JT, Howard SS, Duckett JW, editors. Prostatitis. Adult and pediatric urology. 1991. St. Louis: Mosby-Year Book;1395–1423.
2. Scalbert A, Johnson IT, Saltmarsh M. Polyphenols: antioxidants and beyond. Am J Clin Nutr. 2005. 81:215S–217S.

3. Rahman I, Biswas SK, Kirkham PA. Regulation of inflammation and redox signaling by dietary polyphenols. Biochem Pharmacol. 2006. 72:1439–1452.

4. Kim IB, Kim DY, Lee SJ, Sun MJ, Lee MS, Li H, et al. Inhibition of IL-8 production by green tea polyphenols in human nasal fibroblasts and a549 epithelial cells. Biol Pharm Bull. 2006. 29:1120–1125.

5. Shoskes DA, Zeitlin SI, Shahed A, Rajfer J. Quercetin in men with category III chronic prostatitis: a preliminary prospective, double-blind, placebo-controlled trial. Urology. 1999. 54:960–963.

6. Fujii H, Sun B, Nishioka H, Hirose A, Aruoma OI. Evaluation of the safety and toxicity of the oligomerized polyphenol Oligonol. Food Chem Toxicol. 2007. 45:378–387.

7. Naslund MJ, Strandberg JD, Coffey DS. The role of androgens and estrogens in the pathogenesis of experimental nonbacterial prostatitis. J Urol. 1988. 140:1049–1053.

8. Seethalakshmi L, Bala RS, Malhotra RK, Austin-Ritchie T, Miller-Graziano C, Menon M, et al. 17 beta-estradiol induced prostatitis in the rat is an autoimmune disease. J Urol. 1996. 156:1838–1842.

9. Lundgren R, Holmquist B, Hesselvik M, Müntzing J. Treatment of prostatitis in the rat. Prostate. 1984. 5:277–284.

11. Wheeler DS, Catravas JD, Odoms K, Denenberg A, Malhotra V, Wong HR. Epigallocatechin-3-gallate, a green tea-derived polyphenol, inhibits IL-1 beta-dependent proinflammatory signal transduction in cultured respiratory epithelial cells. J Nutr. 2004. 134:1039–1044.

12. Birrell MA, McCluskie K, Wong S, Donnelly LE, Barnes PJ, Belvisi MG. Resveratrol, an extract of red wine, inhibits lipopolysaccharide induced airway neutrophilia and inflammatory mediators through an NF-kappaB-independent mechanism. FASEB J. 2005. 19:840–841.
13. Manna SK, Mukhopadhyay A, Aggarwal BB. Resveratrol suppresses TNF-induced activation of nuclear transcription factors NF-kappa B, activator protein-1, and apoptosis: potential role of reactive oxygen intermediates and lipid peroxidation. J Immunol. 2000. 164:6509–6519.

14. Donnelly LE, Newton R, Kennedy GE, Fenwick PS, Leung RH, Ito K, et al. Anti-inflammatory effects of resveratrol in lung epithelial cells: molecular mechanisms. Am J Physiol Lung Cell Mol Physiol. 2004. 287:L774–L783.

15. Robinette CL. Sex-hormone-induced inflammation and fibromuscular proliferation in the rat lateral prostate. Prostate. 1988. 12:271–286.

16. Bernoulli J, Yatkin E, Konkol Y, Talvitie EM, Santti R, Streng T. Prostatic inflammation and obstructive voiding in the adult Noble rat: impact of the testosterone to estradiol ratio in serum. Prostate. 2008. 68:1296–1306.

17. Irani J, Levillain P, Goujon JM, Bon D, Doré B, Aubert J. Inflammation in benign prostatic hyperplasia: correlation with prostate specific antigen value. J Urol. 1997. 157:1301–1303.

18. Shull JD, Gorski J. Regulation of prolactin gene transcription in vivo: interactions between estrogen, pimozide, and alphaergocryptine. Mol Pharmacol. 1990. 37:215–221.
19. Tangbanluekal L, Robinette CL. Prolactin mediates estradiol-induced inflammation in the lateral prostate of Wistar rats. Endocrinology. 1993. 132:2407–2416.

20. Wilson MJ, Woodson M, Wiehr C, Reddy A, Sinha AA. Matrix metalloproteinases in the pathogenesis of estradiol-induced nonbacterial prostatitis in the lateral prostate lobe of the Wistar rat. Exp Mol Pathol. 2004. 77:7–17.

21. Domingue GJ Sr, Hellstrom WJ. Prostatitis. Clin Microbiol Rev. 1998. 11:604–613. discussion 213-6.

22. Arakawa S, Matsui T, Gohji K, Okada H, Kamidono S. Prostatitis--the Japanese viewpoint. Int J Antimicrob Agents. 1999. 11:201–203. discussion 213-6.

23. Donovan DA, Nicholas PK. Prostatitis: diagnosis and treatment in primary care. Nurse Pract. 1997. 22:144–146. 149–156.
24. Fujii H, Yokozawa T, Kim YA, Tohda C, Nonaka G. Protective effect of grape seed polyphenols against high glucose-induced oxidative stress. Biosci Biotechnol Biochem. 2006. 70:2104–2111.

25. Li MH, Jang JH, Sun B, Surh YJ. Protective effects of oligomers of grape seed polyphenols against beta-amyloid-induced oxidative cell death. Ann N Y Acad Sci. 2004. 1030:317–329.

26. Orsilles MA, Depiante-Depaoli M. Oxidative stress-related parameters in prostate of rats with experimental autoimmune prostatitis. Prostate. 1998. 34:270–274.

27. Lou JG, Dong J, Zheng YC, Zhang SM, Xiao WQ, Zhou JF. Increased oxidative stress and damage in patients with chronic bacterial prostatitis. Biomed Environ Sci. 2006. 19:481–486.
28. Pasqualotto FF, Sharma RK, Potts JM, Nelson DR, Thomas AJ, Agarwal A. Seminal oxidative stress in patients with chronic prostatitis. Urology. 2000. 55:881–885.

29. Sharma RK, Pasqualotto FF, Nelson DR, Thomas AJ Jr, Agarwal A. The reactive oxygen species-total antioxidant capacity score is a new measure of oxidative stress to predict male infertility. Hum Reprod. 1999. 14:2801–2807.

30. Rahman I, Gilmour PS, Jimenez LA, MacNee W. Oxidative stress and TNF-alpha induce histone acetylation and NF-kappaB/AP-1 activation in alveolar epithelial cells: potential mechanism in gene transcription in lung inflammation. Mol Cell Biochem. 2002. 234-235:239–248.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download