Abstract
Purpose
A prospective study was planned to determine the relationship between post swim-up acrosome index (AI) evaluation and fertilization outcomes in an in vitro fertilization (IVF) program.
Materials and Methods
Infertile couples who have applied to IVF were admitted into this study when the male partner's sperm concentration was > 20×106/mL and motility > 30%. Pre- and post swim-up semen quality parameters including concentration, motility, sperm morphology and AI were evaluated in a prospective, randomized and blinded fashion. The couples were divided prospectively into 2 groups. In group I (25 couples) 50 000 sperm per oocyte were used for insemination considering post swim-up acrosome index, and in group II (25 couples) 50 000 sperm per oocyte were used for insemination without considering post swim-up acrosome index.
Results
Pre- and post swim-up AI were 30.8 ± 3.4 and 17.8 ± 4.5 in group I, and 31.4 ± 3.6 and 16.3 ± 4.7 in group II (p > 0.05) respectively. The significant improvement in morphology and motility after double wash swim-up procedure has been observed. However, double wash swim-up procedure could not eliminate head and especially acrosomal defects which would directly effect fertilization capacity in conventional IVF program. In group I, 85.3% of oocytes were fertilized, with a 48% pregnancy rate; in group II, 71.0% of oocytes were fertilized, with a pregnancy rate of 20%. Fertilization and pregnancy rates were significantly different (p < 0.05) between the two groups.
In Assisted Reproductive Technology (ART) laboratories, semen quality parameters have been accepted as a good predictor of fertilization capacity and pregnancy outcome beside the oocyte quality. The success of in vitro fertilization (IVF) outcome have been influenced by both male and female factors but semen analysis has always been offered as the initial step for evaluating infertile couples.
Semen quality parameters agreed to be sperm concentration, percentage motility and morphology.1 Several authors aimed at finding sperm criteria that correlate well with fertilization rates.2-4 Therefore, alternative sperm strict morphology criteria have been used effectively to develop thresholds that are predictive of fertilization and pregnancy rates following IVF.1,5,6 In addition to the strict criteria, other modified morphological parameters have been developed, such as cytoplasmic residues7 and acrosome index (AI).8
Semen quality parameters including concentration and percentage motility in initial semen samples had been reported by many investigators1,3,5,6,9,10 but morphologic features of processed semen samples had only been mentioned by Scott et al.11
Processed semen samples are obtained by using semen washing procedures. Semen washing procedures are used to remove seminal plasma, immotile sperm, leukocytes, bacteria and debris in order to select morphologically normal and progressively motile spermatozoa. So many methods including swim-up,12 glass-wool filtration13 and density gradient method14 have been developed by various workers in this field.
As IVF is an expensive and stressful treatment, infertile couples generally want to know the expected chance of achieving a pregnancy. It is difficult to predict IVF outcomes as too many variables are included in IVF treatment. The critical issue is that we can use these parameters as a treatment guide.
The aim of this study was to evaluate the relationship between post swim-up AI and fertilization rates in vitro, which has not previously been investigated and to assess whether post swim-up AI consideration has any relevance in IVF outcomes.
Fifty infertile couples who were attempted for IVF treatment between September 2000-May 2003 in an infertility clinique were accepted in this study. All male partners selected were required to have a concentration of more than 20 million/mL and progressively motile sperm fraction of > 30% in the basic semen analysis to try to minimize the impact of these two variables on the fertilization rate and pregnancy outcome. Female partners in these couples had either tubal infertility or unexplained infertility. The couples were divided prospectively into 2 groups. In group I (25 couples) 50 000 sperm per oocyte were used for insemination considering post swim-up acrosome index, and in group II (25 couples) 50 000 sperm per oocyte were used for insemination without considering post swim-up acrosome index. All couples provided their informed concent for this study.
Semen samples were collected by masturbation in sterile polystyrene cups after 3 to 4 days of abstinence. All samples were allowed to liquefy for at least 30 minutes. Volume, viscosity and pH determinations were made immediately after the liquefaction.
Semen analyses were performed in blinded fashion with regard to the study group. Initial sperm concentration and motility were determined using a Makler counting chamber (0.01 mm2, 0.01 mm deep Sefi-Medical Instruments, Haifa, Israel) under magnification using both 10X and 40X. Assessment of motility were made according to four groups classification including progressively rapid motile sperm (PRMS), progressively slow motile sperm (PSMS), local motile sperm (LMS) and immotile sperm (IS) count.
Several other morphologic variables were subsequently determined; namely percentage of normal sperm morphology (strict criteria);1 percentage of spermatozoa with neck/mid-piece defects; percentage of spermatozoa with tail defects; percentage of spermatozoa with cytoplasmic residues; and percentage of spermatozoa with normal acrosome (AI).
According to the routine double wash swim-up procedure used in the andrology laboratory, semen was mixed gently with an equal volume of washing medium consisted of EBSS (Earl's balanced salt solution)(Sigma Chemical Co. E-2888, London, U.K) supplemented with HEPES (Sigma, H-0887, London, U.K), Purivic acid (Sigma, P-5280, St. Louis, MO, U.S.A) and Penicilin/ streptomycin (Biochrom KG, A-2213, Berlin, Germany). Semen samples were washed twice by centrifugation at 1,250 rpm for 10 minutes. The tube was placed in the incubator at 37℃ for 30 minutes at an angle of 45 degrees and the spermatozoa were allowed to swim-up into the medium. After sperm swim-up procedure sperm motility and concentration were evaluated as described previously for initial semen samples.
For morphological analysis, two slides were carefully prepared for each sample. After liquefaction a droplet of 3 to 5 µL from initial semen sample were smeared onto a clean glass slide, allowed to air dry at room temperature and stained using Spermac stain A, B and C (Fertility Technologies, Inc., Natick, MA, USA). Both before and after swim-up procedure, sperm morphologic features were evaluated in a prospective, blinded fashion under oil immersion X1000 objective magnification and for each semen sample at least 100 but preferably 200 spermatozoa were evaluated.
Spermatozoa were considered normal when the head had a smooth oval configuration with a well defined acrosome involving about 40% to 70% of the sperm head, as well as an absence of neck, midpiece or tail defects. Borgerline forms were counted as abnormal. All defects were collected under 4 main groups including head defects (double-head, small, large, pin-head, tapered, amorphous, pyriform, round and elongated), neck/mid-piece defects (bend, non-axial, amorphous and broken), tail defects (double-tail, long, short, dag, coiled and fractured) and cytoplasmic residues.
Spermatozoon acrosomal morphology was evaluated by light microscopy with the method based on acrosomal size and form as well as staining characteristics.8 Results were expressed as the AI (% normal acrosomes). The evaluation of acrosome morphology uses the same principles that are used for the evaluation of normal sperm morphology, according to the strict criteria. For an acrosome to be regarded as normal it must have a smooth normal oval shape, with the same dimensions as for a normal spermatozoon, and acrosomes must be well defined and should comprise 40-70% of the normal-sized sperm head. A spermatozoon can only be classified as normal if the acrosome is classified as normal. The spermatozoon head may have an abnormal or a normal shaped post-acrosomal region, but the other sperm regions must be strictly normal. As with the routine sperm morphology evaluation, at least 200 spermatozoa in two different slides of the same semen sample were examined.
Ovarian stimulation was accomplished by exogenous gonadotropin administration following a desensitization protocol with long-acting GnRH analoques. Human chorionic gonadotropin (HCG : 10,000 UNITS) was given intramuscularly on the evening of the day the mean diameter of the dominant follicle reached 16 mm. -36 h after injection of HCG oocyte retrival was performed. At 2 hour after oocyte recovery, 50,000 sperm added to each oocyte.
For group I, insemination volume (IV) was calculated by: IV (µL)/oocyte = 50 000×1 000 µL/sperm concentration (post swim-up) χAI (% normal)(post swim-up)
For group II insemination volume was calculated by: IV (µL)/oocyte=50 000×1 000 µL/sperm concentration (post swim-up) χ% motility(post swim-up)
Assesment of fertilization was performed 18-20 h after insemination and the presence of two pronuclei was recorded as a sign of fertilization. The fertilized oocytes were transferred to fresh medium and cultured for another 24 h. At 42-44 h after insemination, up to three embryos with the best morphology were transferred to the uterus of the female partners.
Fertilization rate per patient was calculated by dividing the total number of oocytes retrieved, and pregnancy rate per embryo transfer was calculated by dividing the number of pregnancies by the total number of embryo transfers for each group.
Basic descriptive statistics, means, standard deviations (SD), medians and ranges, were calculated for the two groups. Quantitative data are presented as mean ± SD. Statistical analysis was carried out using SPSS software.
Comparison of pre- and post swim-up semen parameters was made using the non-parametric Wilcoxon signed-rank test. p-value of < 0.05 was defined as statistically significant.
As it was not possible to determine precisely the number of MII oocytes in IVF, where they were cumulus-enclosed, all calculations were made considering the number of collected cumulus-oocyte complexes.
General descriptive statistics were performed for each group separately, and Student's t-test was used to compare the fertilization and pregnancy rates of two groups. A value of < 0.05 was chosen as the limit for statistical significance.
Data were collected from 50 couples. Pre- and post swim-up semen parameters were determined for each male partner, as couples were randomly divided into two groups. Fertilization rates and pregnancy rates were evaluated for group I and group II.
For all male partners (n = 50) the mean sperm concentration was 80.4 ± 32.4 million/mL and after double wash swim-up procedure, the concentration dramatically decreased to 44.6 ± 22.5 million/mL (Fig. 1). When sperm motility was compared before and after the procedure, the mean percentage (MP) of progressively rapid motile sperm was 14.8 ± 5.8%, the MP of progressively slow motile sperm was 27.6 ± 3.7% in the initial semen samples, the MP of progressively rapid motile sperm increased to 41.6 ± 5.1% and the MP of progressively slow motile sperm increased to 44.6 ± 4.8% respectively. After sperm preparation technique, the MP of local motile sperm decreased from 8.6 ± 2.9% to 5.0 ± 5.0% and the MP of immotile sperm decreased from 48.8 ± 6.1% to 8.8 ± 5.1%. A comparison of the change in the mean sperm concentration and the mean percentage of motility revealed statistically significant difference (p < 0.001) (Fig. 2).
In these partners, before the swim-up procedure, morphologic features reported as the MP of normal forms 7.4 ± 2.9% though after the swim-up procedure, the MP of normal forms increased up to 13.6 ± 4.1% (Fig. 3). Comparison of these values revealed statistically high significance (p < 0.001).
The mean percentage of head abnormalities increased from 42.0 ± 3.5% to 62.5 ± 5.5%, the MP of cytoplasmic residues increased from 4.9 ± 1.9% to 12.6 ± 4.6%. However the MP of neck/mid-piece abnormalities decreased from 21.8 ± 2.5% to 7.6 ± 5.1% and the MP of tail abnormalities decreased from 23.6 ± 3.6% to 3.4 ± 2.8% respectively in male partners. Comparisons of the changes in the mean percentage of morphology were highly significant (p < 0.001) (Fig. 3).
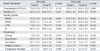
The mean (± SD) age of females in group I and group II was 32.0 ± 4.1 and 30.6 ± 4.6 years, respectively (p < 0.005). For female partners in group I, the mean number of oocytes retrieved and inseminated was 10.4 ± 5.3 (range 3 to 23). In group II, the mean number of oocytes retrieved and inseminated was 7.9 ± 3.5 (range 3 to 20) (Table 1).
Pre- and post swim-up semen parameters for each couple and group were calculated, and there was no significant difference in pre and post swim-up sperm morphology and motility between group I and II (Table 2).
Pre- and post swim-up AI were evaluated further and used for insemination volume calculation in group I. Pre swim-up AI was 30.8 ± 3.4 in group I, and 31.4 ± 3.6 in group II (p > 0.05). Post swim-up AI was 17.8 ± 4.5 in group I, and 16.3 ± 4.7 in group II (p > 0.05) (Table 1).
The mean fertilization rate was 85.3% in group I and 71.0% in group II respectively (p < 0.05).
The pregnancy rate per embryo transfer (ET) was 48% in group I and 20% in group II. With regard to the pregnancy rates there was a significant difference between group I and group II (p < 0.05). The fertilization rates and pregnancy rates are shown in Table 1.
The results of the current study demonstrate that swim-up procedure improves sperm motility and morphology. However, the procedure could not eliminate head and acrosome defects so consideration post swim-up AI for insemination volume calculations seems to be mandatory. Post swim-up semen parameters, including especially AI, correlated with high fertilization and pregnancy rates. We propose that post swim-up AI consideration for insemination volume might directly affect the result of IVF outcome since there were not any significant differences between group I and II with respect to post swim-up total mobile sperm count and AI.
The role of traditional semen parameters (including sperm concentration, motility and morphology) as described in the World Health Organization (WHO, 1992) manual15 had only a limited predictive value as there is a lack of information on the sperm fertilization capacity for the in vivo situation.16
All male partners (n = 50) included in this study have a normal count (> 20 million/mL) and motility (> 30%) as Kruger et al.1 reported a well maintained fertilization rate with a concentration above 20 million/mL and percentage motility above 30%. Although the mean percentage of sperm concentration significantly decreased, the mean percentage of sperm motility increased which might directly effect the fertilization rate and if abnormal morphologic features were not evaluated, these patients could be considered fertile.
It has been commonly agreed that if morphology is not evaluated with care, a diagnosis of unexplained infertility can be made incorrectly and lead to much expectations for both the couple and physician. Kruger's et al.1,5,6 had demonstrated the predictive value of sperm morphology on IVF outcome and also suggested that if evaluation of normal sperm morphology is done using strict criteria, this parameter has an excellent value and it was shown that there was suboptimal fertilization when sperm morphology was < 14% and the lowest levels of fertilization were observed at values of < 4%. After these articles, strict criteria are more often used to evaluate morphologic features than the criteria laid down by World Health Organization.3,11,17,18 Semen parameters included in this study has been adjusted to conform with the strict criteria as introduced by Kruger et al.1 However, the strict criteria for normal sperm morphology were reported to be lacking in accuracy as oocyte fertilization and pregnancy rates in a group of men with < 14% normal morphology were not significantly different from those in a group with > 14% normal morphology.19
Although many investigators pointed out the correlation between sperm morphology and fertilization and pregnancy rates, morphological assessments were being done in initial semen samples but in IVF programmes, processed semen samples were used for actual oocyte inseminations instead of unprocessed semen samples.
The significant improvement in morphology and motility after double wash swim-up procedure have been reported by Scott et al.11 and McDowell et al.20 The results of our study also revealed the significant improvement in these parameters in processed semen samples, whereas double wash swim-up procedure could not eliminate head and especially major acrosome defects which would directly effect fertilization capacity in conventional IVF programmes. Sperm populations with high incidence of head and acrosome defects demonstrated reduced IVF potential (Mashiach et al.21) and recognized as major sperm defects affecting fertilization capacity.22 Similar light microscopic observations have been described by Jeulin et al.23
Acrosome defects are taken by both strict and WHO criteria as a head defect and is not counted as a separate class. Small heads, round heads, pyriform heads also reveal a disturbed acrosome, but have been assigned to head defects and not to acrosome defects. This is of relevance because spermatozoa with minor aberrations of their head morphology may be able to fertilize, but spermatozoa with major aberrations of their acrosomal morphology are unable to fertilize.
The AI as an additional criteria in the diagnosis of a male's fertility potential was introduced by Menkveld et al.8 but has not yet been entirely used for in vivo diagnostic purposes. The correlation between AI and the fertilization rate after IVF-embryo transfer mentioned in the literature.8,24 Previous studies with AI revealed the relationship with total fertilization failure (TFF), in that an oocyte can only be fertilized when a minimum number of sperm cells with intact acrosomes are present.24 The correlation between AI, the acrosin content of a semen sample and the fertilization rate in IVF, has been mentioned.8 Also, it has already been reported that AI predicts both TFF and occurrence of a pregnancy at a cut-off point of 5%. Also in conventional IVF programmes 50 000-100 000 motile sperm per oocyte were being used for oocyte insemination in which motile spermatozoa were expected to have normal morphologic features.3,6
Although, the most emphasized factor for insemination volume has been the number of motile spermatozoon per unit volume in other studies we aimed to investigate if the number of acrosome intact spermatozoon per unit volume has any important effect on the fertilization and pregnancy rates. According to our calculation formula, the insemination volume of group I was larger than that of group II. However, the insemination volume can be different even among the couples in conventional IVF programmes so we suggested that the insemination volume may not have direct effect on the outcomes.
In the present study, post swim-up semen parameters were used to achieve high fertilization and pregnancy rates especially including post swim-up AI calculations and at the same time, to exclude as many couples as possible from being pronounced as infertile, with the subsequent unnecessary treatments and possible sociological problems.
The result of this study suggest that underestimated major acrosomal defects can also be a reason for total fertilization failure outcomes in conventional IVF programmes so acrosome content of spermatozoon inseminated should be evaluated after swim-up procedure. In conclusion, post swim-up AI consideration, after accounting for post swim-up sperm concentration, well correlates with high fertilization and pregnancy rates. Also larger study groups might improve the predictive value of the post swim-up AI, making it a necessary criteria for IVF treatment of selected infertile couples.
Figures and Tables
Fig. 1
The mean sperm concentrations (χ106/mL) before and after double wash swim-up procedure revealed statistically significant difference (p < 0.001).
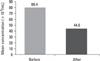
Fig. 2
Comparison of the mean percentage (MP) of motility double wash swim-up procedure was statistically significant (p < 0.001). MP of progressively rapide motile sperm (PRMS), MP of progressively slow motile sperm (PSMS) highly increased whereas MP of locally motile sperm (LMS) and MP of immotile sperm (IS) dramatically decreased after swim-up procedure.
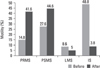
Fig. 3
Comparison of the changes in the mean percentage (MP) of morphology were highly significant (p < 0.001). Comparison of MP of midpiece and tail abnormalities revealed decrease after double wash swim-up procedure whereas comparison of MP of head/acrosome abnormalities and cytoplasmic residues revealed increase after the procedure.

References
1. Kruger TF, Menkveld R, Stander FS, Lombard CJ, Van der Merwe JP, van Zyl JA, et al. Sperm morphologic features as a prognostic factor in in vitro fertilization. Fertil Steril. 1986. 46:1118–1123.

2. Mashiach R, Fisch B, Eltes F, Tadir Y, Ovadia J, Bartoov B. The relationship between sperm ultrastructural features and fertilizing capacity in vitro. Fertil Steril. 1992. 57:1052–1057.

3. Enginsu ME, Dumoulin JC, Pieters MH, Bras M, Evers JL, Geraedts JP. Evaluation of human sperm morphology using strict criteria after Diff-Quik staining: correlation of morphology with fertilization in vitro. Hum Reprod. 1991. 6:854–858.

4. Oehninger S, Mahony M, Ozgür K, Kolm P, Kruger T, Franken D. Clinical significance of human sperm-zona pellucida binding. Fertil Steril. 1997. 67:1121–1127.
5. Kruger TF, Acosta AA, Simmons KF, Swanson RJ, Matta JF, Veeck LL, et al. New method of evaluating sperm morphology with predictive value for human in vitro fertilization. Urology. 1987. 30:248–251.

6. Kruger TF, Acosta AA, Simmons KF, Swanson RJ, Matta JF, Oehninger S. Predictive value of abnormal sperm morphology in in vitro fertilization. Fertil Steril. 1988. 49:112–117.

7. Gomez E, Buckingham DW, Brindle J, Lanzafame F, Irvine DS, Aitken RJ. Development of an image analysis system to monitor the retention of residual cytoplasm by human spermatozoa: correlation with biochemical markers of the cytoplasmic space, oxidative stress and sperm function. J Androl. 1996. 17:276–287.
8. Menkveld R, Rhemrev JP, Franken DR, Vermeiden JP, Kruger TF. Acrosomal morphology as a novel criterion for male fertility diagnosis: relation with acrosin activity, morphology (strict criteria) and fertilization in vitro. Fertil Steril. 1996. 65:637–644.

9. Francavilla F, Romano R, Santucci R, Poccia G. Effect of sperm morphology and motile sperm count on outcome of intrauterine insemination in oligozoospermia and/or asthenozoospermia. Fertil Steril. 1990. 53:892–897.

10. Sanchezn Sarmiento CA, Coetzee K, Kruger TF, van der Merwe JP, Stander FS, et al. Comparison between swim-up and glass wool column filtration of human semen in a gamete intrafallopian transfer program. Arch Androl. 1996. 36:155–160.

11. Scott RT Jr, Oehninger SC, Menkveld R, Veeck LL, Acosta AA. Critical assessment of sperm morphology before and after double wash swim-up preparation for in vitro fertilization. Arch Androl. 1989. 23:125–129.

12. Lopata A, Patullo MJ, Chang A, James B. A method for collecting motile spermatozoa from human semen. Fertil Steril. 1976. 27:677–684.

13. Paulson JD, Polakoski KL. A glass wool column procedure for removing extraneous material from the human ejaculate. Fertil Steril. 1977. 28:178–181.

14. Gorus FK, Pipeleers DG. A rapid method for the fractionation of human spermatozoa according to their progressive motility. Fertil Steril. 1981. 35:662–665.

15. World Health Organisation. WHO laboratory manual for the examination of human semen and sperm-cervical mucus interaction. 1992. 3rd ed. Cambridge: Cambridge University Press.
16. Duncan WW, Glew MJ, Wang XJ, Flaherty SP, Matthews CD. Prediction of in vitro fertilization rates from semen variables. Fertil Steril. 1993. 59:1233–1238.
17. Kobayashi T, Jinno M, Sugimura K, Nozawa S, Sugiyama T, Iida E. Sperm morphological assessment based on strict criteria and in-vitro fertilization outcome. Hum Reprod. 1991. 6:983–986.
18. Enginsu ME, Pieters MH, Dumoulin JC, Evers JL, Geraedts JP. Male factor as determinant of in-vitro fertilization outcome. Hum Reprod. 1992. 7:1136–1140.
19. Morgentaler A, Fung MY, Harris DH, Powers RD, Alper MM. Sperm morphology and in vitro fertilization outcome: a direct comparison of World Health Organization and strict criteria methodologies. Fertil Steril. 1995. 64:1177–1182.

20. McDowel JS, Veeck LL, Jones HW Jr. Analysis of human spermatozoa before and after processing for in vitro fertilization. J In Vitro Fert Embryo Transf. 1985. 2:23–26.

21. Mashiach R, Fisch B, Eltes F, Tadir Y, Ovadia J, Bartoov B. The relationship between sperm ultrastructural features and fertilizing capacity in vitro. Fertil Steril. 1992. 57:1052–1057.

22. Bartoov B, Eltes F, Langsam J, Snyder M, Fisher J. Structural studies in morphological assessment of human spermatozoa. Int J Androl. 1982. 5:81–96.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download