Abstract
Chitin, the second most abundant polysaccharide in nature after cellulose, consist exoskeleton of lower organisms such as fungi, crustaceans and insects except mammals. Recently, several studies evaluated immunologic effects of chitin in vivo and in vitro and revealed new aspects of chitin regulation of innate and adaptive immune responses. It has been shown that exogenous chitin activates macrophages and other innate immune cells and also modulates adaptive type 2 allergic inflammation. These studies further demonstrate that chitin stimulate macrophages by interacting with different cell surface receptors such as macrophage mannose receptor, toll-like receptor 2 (TLR-2), C-type lectin receptor Dectin-1, and leukotriene B4 recepptor (BLT1). On the other hand, a number of chitinase or chitinase-like proteins (C/CLP) are ubiquitously expressed in the airways and intestinal tracts from insects to mammals. In general, these chitinase family proteins confer protective functions to the host against exogenous chitin-containing pathogens. However, substantial body of recent studies also set light on new roles of C/CLP in the development and progression of allergic inflammation and tissue remodeling. In this review, recent findings on the role of chitin and C/CLP in allergic inflammation and tissue remodeling will be highlighted and controversial and unsolved issues in this field of studies will be discussed.
Chitin, β-(1-4)-poly-N-acetyl D-glucosamine, is widely distributed in nature and is the second most abundant polysaccharide in nature after cellulose. It is found in the cell walls of bacteria and fungi, mushrooms, exoskeleton of crustaceans (crabs, shrimp, etc.) and insects, the microfilarial sheath of parasitic nematodes, and the lining of the digestive tracts of many insect.1-8 In these locations, chitin is used by the chitin-containing organisms to protect it from the harsh conditions in its environment and host anti-parasite/pathogen immune responses. The mammalian counterpart of chitin has not been described. Despite its ubiquity, chitin does not accumulate in the environment mainly because chitinolytic bacteria or saprophytes efficiently recycle most of chitin.9 As some chitin derivatives are known to be non-toxic, non-allergenic, biodegradable, and biocompatible, a number of prostheses such as artificial skin, contact lenses, surgical stitches have been produced from chitin derivatives and are widely used in medical practice.10 Thus, it is very common for humans to be exposed to chitin/chitin derivatives in daily life.
Although mammals cannot synthesize or metabolize chitin, a number of chitinolytic enzymes [true chitinases, e.g., chitotriosidases, acidic mammalian chitinase (AMCase)] or chitin-binding proteins [chitinase-like proteins (CLPs), e.g., Ym-1, Ym-2, breast regression protein 39 (BRP-39, chondrocyte protein-39)] were discovered in mammals.5,11 Many of the chitinase family proteins are constitutively expressed in macrophages and epithelial cells of the lung and digestive tracts, consisting of the body's first line of defense against exogenous agents including chitin-containing pathogens.12,13 Interestingly, some of the chitinases or CLPs (C/CLPs) have been reported to be expressed in a inducible manner in certain inflammatory and allergic conditions.14-16 Increased expression of AMCase in the lung was reported in the development of Th2 inflammation in allergic animal models and the human asthmatic airway.14 Genetic studies in human populations have shown linked polymorphisms in AMCase to asthma susceptibility in children, suggesting that inherent defects in this chitinase could underlie airway inflammation and allergic responses.17 Recently, a similar role has been proposed with a chitinase-like protein YKL-40. The serum and tissue expression of YKL-40 was significantly correlated with the severity of asthma, and genetic association of serum YKL levels and asthmatic airways has been identified.18,19 These studies also raise the possibility that the chitin, as a major binding partner of C/CLPs in mammals, might play an important role as a modulator (initiator or enhancer) of allergic immune responses, in that C/CLPs play a critical role. However, interactions between chitin and C/CLPs have not been adequately studied.
Two decades ago, a number of studies demonstrated that chitin and chitin derivatives stimulated macrophages to produce cytokines that confer non-specific host resistance to bacterial and viral infections and anti-tumor activity.19-24
Since then, a number of studies describing more specific immunologic activities of chitin have been reported. Shibata et al. re-evaluated the immunological effects of chitin in vivo and in vitro using phagocytosable small-sized chitin particles that demonstrated significant priming effects of chitin particles in alveolar macrophages and NK cells in mice.25 They showed intravenous administration of fractionated chitin particles (1 to 10 µm) into the lung activates alveolar macrophages to express cytokines such as IL-12, tumor necrosis factor (TNF)-α, and IL-18, leading to INF-γ production mainly by NK cells.25 Subsequent studies by the same group of investigators demonstrated that the cytokine production was through a mannose-receptor mediated phagocytosis process.26 The macrophage plasma membrane mannose receptors serve to mediate the internalization of the chitin particles that, eventually, are degraded by the lysozyme and N-acetyl-β-glucosaminidase in the macrophages of human and experimental animals.27 Those studies were the first demonstration of the direct interactions between chitin and cell surface receptors and raised the possibility that chitin uses specific signaling pathways in immune regulation.
Recently, intriguing direct in vivo immune modulatory function of chitin has been described.28 The investigators administrated chitin beads directly into the lungs of mice expressing a green fluorescent protein (GFP)-enhanced transcript of IL-4 (4get mice) via intranasal application. Within several hours after chitin exposure, IL-4 GFP positive cells, in particular eosinophils (GFP+, siglec F+) and basophils (GFP+, IgE+, cKit-), were recruited to the lungs of these mice. The chitin-induced eosinophil recruitment was shown to be dependent on leukotriene B4, because eosinophilic recruitment was significantly decreased in the leukotriene B4 receptor null mice (BLT1). They further demonstrated that chitin alternatively activates alveolar macrophages and macrophage response was critical event in recruitment of eosinophils because depletion of macrophages by clodronate liposome treatment prevented recruitment of eosinophils. These studies raise the possibility that chitin can be directly involved in the generation of allergic responses and provide another clues to explain high asthma frequency in the workers predicted to have high environmental exposure of chitin.29,30
As described above, there are multiple evidences indicating that chitin is a potent innate immune stimulator of macrophages and other innate immune cells. This raises the possibility that chitin could affect allergen-induced adaptive type II responses as well. Generally, type I cytokines are produced by innate immune cells and it has been shown that type I cytokines down-regulate type 2 allergic immune responses.31 In addition, the administration of IFN-γ or IL-12 significantly inhibited Th2 driven inflammatory responses in allergic animal models.32,33 Thus, it is reasonable to speculate that chitin could negatively modulate allergen-induced type 2 inflammatory responses if chitin does stimulate type I cytokines production. Several studies strongly support this contention. Shibata et al. has elegantly demonstrated that orally given chitin significantly down regulates allergen-induced IgE production and lung inflammation in a ragweed-immunized allergic animal model.31 The allergen-stimulated Th2 cytokines, such as IL-4, IL-5, and IL-10 production was significantly inhibited by the presence of chitin in spleen cell culture. They further demonstrated that IFN-γ produced by NK cells and ragweed-specific Th1 cells was responsible for the inhibition of allergen-induced Th2 cytokine production.31 In a separate study, the same group of investigators have shown that chitin is a strong Th1 adjuvant that up-regulates heat-killed Mycobacterium bovis Calmette-Guerin bacillus (HK-BCG)-induced Th1 immunity, but down regulates mycobacterial protein (MPB-39)-induced Th2 immunity.34 The Th1 adjuvant effect of chitin microparticles (CMP) in inducing viral specific immunity has also been reported.35 Later studies by Strong et al. further demonstrated that direct intranasal application of chitin microparticles into the lung also significantly down-regulated allergic response to Dermatophagoids pteronyssinus (Der p) and Aspergillus fumigatus in a murine model of allergy.36 The chitin treatment substantially reduced these allergen-induced serum IgE levels, peripheral eosinophilia, airway hyper-responsiveness, and lung inflammation. They noted the elevation of Th1 cytokines IL-12, IFN-γ and TNF-α and reduction in IL-4 production in the chitin-treated mice compared to sham controls. Similarly, intranasal application of water soluble chitosan also significanlty attenuated Dermatophagoids farinae (Der f)-induced lung inflammation and mucus production.36 Subsequent studies by Ozdemir et al. further demonstrated that application of microgram quantities of chitin microparticles had a beneficial effect in preventing and treating histopathologic changes in the airways of asthmatic mice.37 All these studies strongly support the contention that chitin negatively regulate the development of adaptive type 2 allergic responses. As a regulatory mechanism, down regulation of allergen-induced arginase I and thymic stromal lymphopoietin (TSLP) expression in the bronchial epithelium was suggested.38 The significant role of TSLP and arginase I in Th2 polarization and tissue remodeling process has been previously described, respectively.39,40 From the clinical point of view, the regulatory function of chitin on Th2 adaptive immune response is therapeutically important because it can be applied to control a variety of type 2 allergic diseases.
Although many studies strongly suggest that chitin or chitin derivatives enhance Type I immunity while suppressing Type II inflammatory responses, a number of issues remain to be clarified to generalize these effects. The animal models that were used to assay chitin effects in these studies may represent only a specific type of allergic response. This needs to be tested in other type 1 as well as type 2 allergic animal models such as animal models using Ovalbumin (OVA) as a immunizing allergen.41,42 As discussed in the above section, in vivo chitin also stimulated innate immune cells, such as eosinophils and basophils, the cells that are closely associated with allergic responses. Type 2 cytokines produced from these innate immune cells can further enhance allergen-induced inflammatory and tissue responses. These studies suggest that in vivo regulation of chitin on adaptive Th2 immune response may not be simple or uni-directional. In addition, a Th2 adjuvant effect of chitin in a Th2 allergic animal model has been observed (unpublished observations). When viewed in combination, these studies demonstrate that chitin or chitin derivatives have complex in vivo regulatory mechanisms on adaptive immune responses and this necessitates clarification for future clinical applications of chitin in allergic diseases.
The chitinases Der p 15 and Der p 18 originated from house dust mites have been identified as major dog allergens43 and also suggested to be allergenic to humans.44 The potential role of endogenous mammalian C/CLPs in allergic immune response only recently been investigated (for review see.5,45 One of the prototypic chitinase AMCase was first noted to be induced during Th2 inflammation through an IL-13-dependent mechanism.14 It was also shown to play an important role in the pathogenesis of Th2 inflammations and IL-13 effector pathway activation.14 In support of this contention, subsequent studies also demonstrated that potential role of AMCase in human asthma,17 ocular allergy,46 and allergic airway inflammation.47 However, in contrary to the mice system, recent studies demonstrated that AMCase expressed in human lung was mostly inactive and chitotriosidase was mainly responsible for the chitinase activity in the lung.48 Thus, whether the chitotriosidase in humans has the same immunologic activity in allergic responses as AMCase in mouse is an interesting question that need to be addressed in further studies. Recently more exciting stories were developed for role of CLPs in allergic diseases. In the mouse allergic models, Ym-1 and Ym-2 was recognized as a allergy-associated protein.44,47,49 In humans, our laboratory demonstrated that levels of YKL-40/BRP-39 in sera and lung tissues were significantly associated with asthma development and even severity of the disease.18 Subsequent extensive population studies also demonstrated that YKL-40 polymorphisms are functionally and genetically associated with asthma patients,19 suggesting an important role of YKL-40/BRP-39 in the pathogenesis of asthma or Th2 allergic diseases. Interestingly, a significant role of YKL-40/BRP-39 in the generation of intestinal bowel disease (IBD) as a active pathogenic mediator in acute colitis has been suggested.13,15 When viewed in combination, these studies provide noble insights on the biological role of C/CLPs in a number of allergic and inflammatory diseases including asthma, asthritis, and rhinosinusitis, and IBD. However, specific functional role of C/CLPs in allergic diseases remains to be defined.
Recently a number of studies suggest that important role of C/CLPs in disease pathogenesis that characterized by pathologic tissue remodeling. Bargagli et al.50 reported that significantly higher activity of chitotriosidase, in serum and BAL of patients with sarcoidosis, especially in those with progressing disease and lung involvement, than in controls. They also showed that the increase of the chitotriosidase activity was specific in sarcoidosis patients because the activity was in normal range in the patients with tuberclosis or with idiopathic pulmonary fibrosis and pulmonary fibrosis associated with systemic sclerosis.50,51 In addition, potential role of chitotriosidase in the evolution of nonalcoholic fatty liver disease such as non-alcoholic steatohepatitis (NASH) has been suggested.52,53 In those studies, Kupffer cells in NASH patients overproduce chitotriosidase contributed to the progression from uncomplicated steatosis to steatohepatitis with progressive fibrosis.53 Several studies also suggested that CLPs such as YKL-40 or mouse Ym-1 or Ym-2 could be involved in tissue remodeling process. Serum YKL-40 was significantly related to the degree of liver fibrosis and staining of YKL-40 antigen was higher in areas with fibrosis, particularly areas with active fibrogenesis.54,55 The serum levels of YKL-40 was correlated with the stage of hepatic fibrosis in patients infected with S. japonicum, further support potential role of YKL-40 in the pathogenesis of fibrogenesis.56 In the animal models that accompanying tissue remodeling process also demonstrated significant changes in the C/CLPs expression at the site of inflammation or remodeling. Th2-inducing pathogens Schistosoma mansoni and Nippostrongylus brasiliensis cause granulomatous inflammation and liver fibrosis in the infested mice. In that model, AMCase and Ym-1 expression were significantly increased along with type 2 cytokines such as IL-13 and IL-4.57 In the mice with pulmonary fibrosis induced by crystalline silica exposure,58 or herpesvirus59 also demonstrated that close associations between expression of C/CLPs and degree of tissue remodeling. However, it is still not clear whether C/CLPs actively participate in the tissue remodeling process or indirectly modulate the process through regulation of other cytokines and/or growth factors. The elevated expression of C/CLPs could only represent specific type of macrophage activation, such as alternative activation of macrophages, that produce a number of potent growth factors capable of inducing tissue remodeling. In this case, C/CLPs only involves in the activation process of macrophages and other cells. Further mechanistic studies using specific gene targeted animal models or transgenic models will be required to define more specific function of C/CLPs in tissue remodeling.
The macrophages response to exogenous chitin is not consistent when similar studies are compared. The earlier studies indicated that direct stimulation of macrophages with chitin or chitin derivatives significantly enhanced the expression of type I pro-inflammatory cytokines such as TNF-α and IL-12 and IFN-γ.21,60 Later, Shibata et al. further supported the contention that chitin stimulates macrophages to express type I cytokines such as TNF-α, IL-18 and IL-12 that resulted in the expression of IFN-γ by NK cells.25,26 IFN-γ is well known cytokine that classically stimulates macrophages.61 However, studies from Reese et al. demonstrated that chitin alternatively activated macrophages (AAM) that were critical for chitin-induced inflammatory responses.28 It has been shown that chitin or chitin derivatives stimulate wound healing via AAM.10 Other studies indicated that chitin directly stimulates macrophages to express TNF-α and IL-17 via TLR-2 and MyD-88-dependent but TLR-4-independent pathways.62 These studies suggest a number of pathways, and cytokines are possibly involved in chitin-induced macrophages activation and cytokine production. Also, the discrepancies in chitin-induced macrophage activation between similar studies were possibly originated from the difference in the experimental system itself. The possibility of other contaminants, such as lipids or β-glucan, was excluded by demonstrating that chitin-induced responses were abolished by the treatment of chitin with chitinase before challenging animals.28 LPS contamination has been also excluded because the chitin-induced response was known to be TLR-4 independent.28,62 However, the chitin preparation (size difference), doses, duration, and route of chitin administration could differently affect inflammatory and tissue responses.9,28 When viewed in combination, it is more reasonable for now to speculate that chitin has the capacity to stimulate macrophages in multiple directions depending on experimental conditions or pathways involved. Chitin can interact with diverse cell surface receptors, such as macrophage mannose receptor, TLR-2 receptors,25,62 and a C-type lectin receptor dectin-1 (unpublished observation). It would be very interesting to define specific chitin-receptor interactions for macrophage activation. The alternative activation of macrophages by chitin could be a secondary event followed by the initial influx of eosinophils or other inflammatory cells expressing type II cytokines. In this scenario, chitin initially activates macrophages to express pro-inflammatory mediators, such as LTB428, that recruit eosinophils and other innate immune cells expressing type II cytokines. Then, the macrophages can be alternatively activated by the cytokines expressed by those inflammatory cells. To understand the nature of chitin-induced immune regulation, further characterization of underlying mechanisms leading to distinct macrophage activation will be required in future studies.
Epithelial cells and other antigen presenting cells (APCs) in the airways or the gut are often exposed to a variety of exogenous allergens and potentially chitin-containing pathogens by inhalation or ingestion. Epithelial cells endogenously express chitinases and chitin binding proteins or their expression is significantly induced by allergenic stimulation.14,44,49 The specific role of epithelial cells in chitin-induced immune response has not been carefully evaluated, and the effects of chitin on innate immune cells other than macrophages and NK cells remains largely unknown. As epithelial and dendritic cells are key players in allergen (or pathogen) recognition and processing, it is important to define the response of these cells to chitin to understand the immune regulatory function of chitin in vivo. Because dendritic cells express a number of toll-like receptors and C-type lectin receptors endogenously and in a inducible manner like macrophages,63,64 it is reasonable to speculate that exogenous chitin can directly interact with dendritic cells, and modulate functional phenotypes that lead to specific polarized T cell responses. It has been shown that when airway epithelial cells are exposed to allergen or injury, several alarmins, such as TSLP, along with pro- and anti- inflammatory cytokines are produced from epithelial cells, that could affect dendritic cell phenotypes and functions.39 Interestingly, chitosan has been shown to down-regulate TSLP expression from allergen-stimulated epithelial cells,38 suggesting potentially important roles of epithelial cells and dendritic cells in the immunological activity of chitin. Cytokines, chemokines and other mediators produced from chitin stimulated epithelial cells or dendritic cells will be interesting targets for future studies.
Although several in vivo and in vitro studies have provided substantial evidence that chitin uses certain receptors expressed on the surface of macrophages, the exact interacting molecule(s) has not yet been defined. As discussed in the previous section, although chitin is not produced in mammals, many chitinases or CLPs are endogenously expressed in mammals. Chitinases and CLPs belong to family 18 glycosyl hydrolase members which contain a catalytic domain and a chitin binding domain.5,65 The physical binding properties between chitin and a CLP have been characterized in previous studies.65-67 However, the exact role of these chitinase or chitinase-like proteins in the regulation of chitin-induced inflammatory and immune regulatory function remains to be determined. Previous studies demonstrated that the expression of a number of chitinases and CLPs were induced in allergic animal models and humans with allergic inflammation. However, it is undetermined whether the chitinase or CLPs expression is induced or not by in vivo chitin stimulation. Recently, Reese et al. reported that after in vivo administration of chitin into the lungs of chitinase overexpressing transgenic mice, chitin-induced eosinophil and basophil recruitment was abolished.28 These studies suggest a protective role of chitinases against chitin or chitin-containing pathogens such as parasites. However, previous studies from our laboratory and others demonstrated significant induction of chitinases and CLPs in an animal model of allergy using non-chitin containing allergen such as OVA.14,49 Chitinases and CLPs are also impressively induced in the lung of IL-13 overexpressing transgenic mice, and specific inhibition of chitinase activity in these animal models using chemical (allosamidine) or neutralization antibody against specific chitinases, significantly reduced OVA- or IL-13-induced inflammation.14 These studies suggest that endogenous chitinases or CLPs could be involved in general host response, irrespective of chitin. When viewed in combination, chitin or allergen modulated the expression of chitinases or chitin-binding proteins, and these chitinases may have a direct protective role against chitin by degrading the chitin itself, but also may function to amplify inflammatory responses against chitin or other allergens. The question as to what kinds of chitinases or chitinase-like molecules are induced in response to exogenous chitin, and how this endogenous chitinase family of proteins interacts with chitin in immune responses, is still waiting to be answered.
A number of studies indicate that chitin and chitin derivatives have diverse biological activities. Not only the abundance of chitin in the environment or the growing use of chitin or chitin derivatives in various biomedical fields but also the potent immunologic activity of chitin has prompted us to consider chitin as a potential pro- or anti-allergenic molecule. Recent studies demonstrated that chitin primarily stimulate innate immune cells to generate type I or type 2 inflammatory responses depending on the chitin size or composition and/or ways of chitin administration (Fig. 1). In vivo chitin also directly or indirectly modulates allergen-induced adaptive inflammation through a number of ways (Fig. 1). However, exact in vivo immune regulatory effects of chitin has been controversial and the underlying mechanisms of chitin regulation of allergic response has not been clearly defined. Also a number of recent studies strongly suggest that C/CLPs are involved in the development or progression of allergic diseases and tissue remodeling. Further characterization on the interactions between chitin and C/CLPs, the specific role of endogenous C/CLPs in allergic immune responses and tissue remodeling, and underlying signaling pathways will be required to understand the exact biological role of chitin, C/CLPs in the pathogenesis of allergic and inflammatory diseases.
Figures and Tables
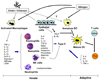 | Fig. 1Chitin stimulation of innate immune cells and regulation of adaptive allergic immune responses. Chitin or chitin derivatives activate macrophages to express a number of pro-inflammatory cytokines, chemokine (e.g., GCP-2) and other mediators (LTB4) via macrophage mannose receptor, TLR-2, and C-type lectin receptor. Then the eosinophils, basophils and neutrophils that are recruited by these inflammatory mediators present type II allergic responses by secretion of Th2 cytokines (IL-4, IL-5, IL-13) and other allergic mediators (histamines, peroxidases). Thus, chitin-induced eosinophils and basophils further enhance allergen-induced adaptive Th2 inflammatory responses. On the other hand, many studies also support that chitin activates macrophages, NK cells and neutrophils to produce type I cytokines (TNF-α, IL-12, IL-1β and IFN-γ), and suppress allergen-induced adaptive type II immune responses. Chitosan down-regulate allergen-induced TSLP expression from epithelial cells, and inhibits Th2 polarization by TSLP-dendritic cell interaction. The direct effect of chitin and chitin derivatives on dendritic cells remains to be determined. |
References
1. Araujo AC, Souto-Padrón T, de Souza W. Cytochemical localization of carbohydrate residues in microfilariae of Wuchereria bancrofti and Brugia malayi. J Histochem Cytochem. 1993. 41:571–578.

2. Boot RG, Blommaart EF, Swart E, Ghauharali-van der Vlugt K, Bijl N, Moe C, et al. Identification of a novel acidic mammalian chitinase distinct from chitotriosidase. J Biol Chem. 2001. 276:6770–6778.

3. Boot RG, Renkema GH, Verhoek M, Strijland A, Bliek J, de Meulemeester TM, et al. The human chitotriosidase gene. Nature of inherited enzyme deficiency. J Biol Chem. 1998. 273:25680–25685.
4. Debono M, Gordee RS. Antibiotics that inhibit fungal cell wall development. Annu Rev Microbiol. 1994. 48:471–497.

5. Elias JA, Homer RJ, Hamid Q, Lee CG. Chitinases and chitinase-like proteins in T(H)2 inflammation and asthma. J Allergy Clin Immunol. 2005. 116:497–500.

6. Fuhrman JA, Piessens WF. Chitin synthesis and sheath morphogenesis in Brugia malayi microfilariae. Mol Biochem Parasitol. 1985. 17:93–104.

7. Neville AC, Parry DA, Woodhead-Galloway J. The chitin crystallite in arthropod cuticle. J Cell Sci. 1976. 21:73–82.

8. Shahabuddin M, Kaslow DC. Plasmodium: parasite chitinase and its role in malaria transmission. Exp Parasitol. 1994. 79:85–88.

9. Burton OT, Zaccone P. The potential role of chitin in allergic reactions. Trends Immunol. 2007. 28:419–422.

10. Muzzarelli RA. Human enzymatic activities related to the therapeutic administration of chitin derivatives. Cell Mol Life Sci. 1997. 53:131–140.

11. Myles O, Wortmann GW, Cummings JF, Barthel RV, Patel S, Crum-Cianflone NF, et al. Visceral leishmaniasis: clinical observations in 4 US army soldiers deployed to Afghanistan or Iraq, 2002-2004. Arch Intern Med. 2007. 167:1899–1901.

12. Homer RJ, Zhu Z, Cohn L, Lee CG, White WI, Chen S, et al. Differential expression of chitinases identify subsets of murine airway epithelial cells in allergic inflammation. Am J Physiol Lung Cell Mol Physiol. 2006. 291:L502–L511.

13. Mizoguchi E. Chitinase 3-like-1 exacerbates intestinal inflammation by enhancing bacterial adhesion and invasion in colonic epithelial cells. Gastroenterology. 2006. 130:398–411.

14. Zhu Z, Zheng T, Homer RJ, Kim YK, Chen NY, Cohn L, et al. Acidic mammalian chitinase in asthmatic Th2 inflammation and IL-13 pathway activation. Science. 2004. 304:1678–1682.

15. Kawada M, Hachiya Y, Arihiro A, Mizoguchi E. Role of mammalian chitinases in inflammatory conditions. Keio J Med. 2007. 56:21–27.

16. Johansen JS. Studies on serum YKL-40 as a biomarker in diseases with inflammation, tissue remodelling, fibroses and cancer. Dan Med Bull. 2006. 53:172–209.
17. Bierbaum S, Nickel R, Koch A, Lau S, Deichmann KA, Wahn U, et al. Polymorphisms and haplotypes of acid mammalian chitinase are associated with bronchial asthma. Am J Respir Crit Care Med. 2005. 172:1505–1509.

18. Chupp GL, Lee CG, Jarjour N, Shim YM, Holm CT, He S, et al. A chitinase-like protein in the lung and circulation of patients with severe asthma. N Engl J Med. 2007. 357:2016–2027.

19. Ober C, Tan Z, Sun Y, Possick JD, Pan L, Nicolae R, et al. Effect of variation in CHI3L1 on serum YKL-40 level, risk of asthma, and lung function. N Engl J Med. 2008. 358:1682–1691.

20. Nishimura K, Nishimura S, Nishi N, Saiki I, Tokura S, Azuma I. Immunological activity of chitin and its derivatives. Vaccine. 1984. 2:93–99.

21. Nishimura S, Nishi N, Tokura S, Nishimura K, Azuma I. Bioactive chitin derivatives. Activation of mouse-peritoneal macrophages by O-(carboxymethyl)chitins. Carbohydr Res. 1986. 146:251–258.

22. Suzuki K, Okawa Y, Hashimoto K, Suzuki S, Suzuki M. Protecting effect of chitin and chitosan on experimentally induced murine candidiasis. Microbiol Immunol. 1984. 28:903–912.

23. Ellouz F, Adam A, Ciorbaru R, Lederer E. Minimal structural requirements for adjuvant activity of bacterial peptidoglycan derivatives. Biochem Biophys Res Commun. 1974. 59:1317–1325.

24. Azuma I, Sugimura K, Taniyama T, Yamawaki M, Yamamura Y. Adjuvant activity of mycobacterial fractions: adjuvant activity of synthetic N-acetylmuramyl-dipeptide and the related compounds. Infect Immun. 1976. 14:18–27.

25. Shibata Y, Metzger WJ, Myrvik QN. Chitin particle-induced cell-mediated immunity is inhibited by soluble mannan: mannose receptor-mediated phagocytosis initiates IL-12 production. J Immunol. 1997. 159:2462–2467.
26. Shibata Y, Foster LA, Metzger WJ, Myrvik QN. Alveolar macrophage priming by intravenous administration of chitin particles, polymers of N-acetyl-D-glucosamine, in mice. Infect Immun. 1997. 65:1734–1741.

27. Bourbouze R, Raffi F, Dameron G, Hali-Miraftab H, Loko F, Vilde JL. N-acetyl-beta-D-glucosaminidase (NAG) isoenzymes release from human monocyte-derived macrophages in response to zymosan and human recombinant interferon-gamma. Clin Chim Acta. 1991. 199:185–194.
28. Reese TA, Liang HE, Tager AM, Luster AD, Van Rooijen N, Voehringer D, et al. Chitin induces accumulation in tissue of innate immune cells associated with allergy. Nature. 2007. 447:92–96.

29. Zhang Y, Matsuo H, Morita E. Cross-reactivity among shrimp, crab and scallops in a patient with a seafood allergy. J Dermatol. 2006. 33:174–177.

30. Desjardins A, Malo JL, L'Archevêque J, Cartier A, McCants M, Lehrer SB. Occupational IgE-mediated sensitization and asthma caused by clam and shrimp. J Allergy Clin Immunol. 1995. 96:608–617.
31. Shibata Y, Foster LA, Bradfield JF, Myrvik QN. Oral administration of chitin down-regulates serum IgE levels and lung eosinophilia in the allergic mouse. J Immunol. 2000. 164:1314–1321.
32. Sur S, Lam J, Bouchard P, Sigounas A, Holbert D, Metzger WJ. Immunomodulatory effects of IL-12 on allergic lung inflammation depend on timing of doses. J Immunol. 1996. 157:4173–4180.
33. Gavett SH, O'Hearn DJ, Li X, Huang SK, Finkelman FD, Wills-Karp M. Interleukin 12 inhibits antigen-induced airway hyperresponsiveness, inflammation, and Th2 cytokine expression in mice. J Exp Med. 1995. 182:1527–1536.
34. Shibata Y, Honda I, Justice JP, Van Scott MR, Nakamura RM, Myrvik QN. Th1 adjuvant N-acetyl-D-glucosamine polymer up-regulates Th1 immunity but down-regulates Th2 immunity against a mycobacterial protein (MPB-59) in interleukin-10-knockout and wild-type mice. Infect Immun. 2001. 69:6123–6130.
35. Hamajima K, Kojima Y, Matsui K, Toda Y, Jounai N, Ozaki T, et al. Chitin Micro-Particles (CMP): a useful adjuvant for inducing viral specific immunity when delivered intranasally with an HIV-DNA vaccine. Viral Immunol. 2003. 16:541–547.

36. Strong P, Clark H, Reid K. Intranasal application of chitin microparticles down-regulates symptoms of allergic hypersensitivity to Dermatophagoides pteronyssinus and Aspergillus fumigatus in murine models of allergy. Clin Exp Allergy. 2002. 32:1794–1800.
37. Ozdemir C, Yazi D, Aydogan M, Akkoc T, Bahceciler NN, Strong P, et al. Treatment with chitin microparticles is protective against lung histopathology in a murine asthma model. Clin Exp Allergy. 2006. 36:960–968.

38. Chen CL, Wang YM, Liu CF, Wang JY. The effect of water-soluble chitosan on macrophage activation and the attenuation of mite allergen-induced airway inflammation. Biomaterials. 2008. 29:2173–2182.

39. MacDonald AS, Maizels RM. Alarming dendritic cells for Th2 induction. J Exp Med. 2008. 205:13–17.

40. Liu YJ, Soumelis V, Watanabe N, Ito T, Wang YH, Malefyt Rde W, et al. TSLP: an epithelial cell cytokine that regulates T cell differentiation by conditioning dendritic cell maturation. Annu Rev Immunol. 2007. 25:193–219.

41. Shibaki A, Katz SI. Induction of skewed Th1/Th2 T-cell differentiation via subcutaneous immunization with Freund's adjuvant. Exp Dermatol. 2002. 11:126–134.

42. Lee CG, Hartl D, Matsuura H, Dunlop FM, Scotney PD, Fabri LJ, et al. Endogenous IL-11 signaling is essential in Th2- and IL-13-induced inflammation and mucus production. Am J Respir Cell Mol Biol. 2008. 39:739–746.

43. Weber E, Hunter S, Stedman K, Dreitz S, Olivry T, Hillier A, et al. Identification, characterization, and cloning of a complementary DNA encoding a 60-kd house dust mite allergen (Der f 18) for human beings and dogs. J Allergy Clin Immunol. 2003. 112:79–86.

44. Song HM, Jang AS, Ahn MH, Takizawa H, Lee SH, Kwon JH, et al. Ym1 and Ym2 expression in a mouse model exposed to diesel exhaust particles. Environ Toxicol. 2008. 23:110–116.

45. Donnelly LE, Barnes PJ. Acidic mammalian chitinase--a potential target for asthma therapy. Trends Pharmacol Sci. 2004. 25:509–511.
46. Musumeci M, Bellin M, Maltese A, Aragona P, Bucolo C, Musumeci S. Chitinase levels in the tears of subjects with ocular allergies. Cornea. 2008. 27:168–173.

47. Zhao J, Zhu H, Wong CH, Leung KY, Wong WS. Increased lungkine and chitinase levels in allergic airway inflammation: a proteomics approach. Proteomics. 2005. 5:2799–2807.

48. Seibold MA, Donnelly S, Solon M, Innes A, Woodruff PG, Boot RG, et al. Chitotriosidase is the primary active chitinase in the human lung and is modulated by genotype and smoking habit. J Allergy Clin Immunol. 2008. 122:944–950. e3.

49. Webb DC, McKenzie AN, Foster PS. Expression of the Ym2 lectin-binding protein is dependent on interleukin (IL)-4 and IL-13 signal transduction: identification of a novel allergy-associated protein. J Biol Chem. 2001. 276:41969–41976.

50. Bargagli E, Margollicci M, Nikiforakis N, Luddi A, Perrone A, Grosso S, et al. Chitotriosidase activity in the serum of patients with sarcoidosis and pulmonary tuberculosis. Respiration. 2007. 74:548–552.

51. Bargagli E, Margollicci M, Luddi A, Nikiforakis N, Perari MG, Grosso S, et al. Chitotriosidase activity in patients with interstitial lung diseases. Respir Med. 2007. 101:2176–2181.

52. Malaguarnera L, Rosa MD, Zambito AM, dell'Ombra N, Marco RD, Malaguarnera M. Potential role of chitotriosidase gene in nonalcoholic fatty liver disease evolution. Am J Gastroenterol. 2006. 101:2060–2069.

53. Malaguarnera L, Di Rosa M, Zambito AM, dell'Ombra N, Nicoletti F, Malaguarnera M. Chitotriosidase gene expression in Kupffer cells from patients with non-alcoholic fatty liver disease. Gut. 2006. 55:1313–1320.

54. Johansen JS, Christoffersen P, Møller S, Price PA, Henriksen JH, Garbarsch C, et al. Serum YKL-40 is increased in patients with hepatic fibrosis. J Hepatol. 2000. 32:911–920.

55. Johansen JS, Møller S, Price PA, Bendtsen F, Junge J, Garbarsch C, et al. Plasma YKL-40: a new potential marker of fibrosis in patients with alcoholic cirrhosis? Scand J Gastroenterol. 1997. 32:582–590.

56. Zheng M, Cai WM, Zhao JK, Zhu SM, Liu RH. Determination of serum levels of YKL-40 and hyaluronic acid in patients with hepatic fibrosis due to schistosomiasis japonica and appraisal of their clinical value. Acta Trop. 2005. 96:148–152.

57. Pesce J, Kaviratne M, Ramalingam TR, Thompson RW, Urban JF Jr, Cheever AW, et al. The IL-21 receptor augments Th2 effector function and alternative macrophage activation. J Clin Invest. 2006. 116:2044–2055.

58. Migliaccio CT, Buford MC, Jessop F, Holian A. The IL-4Ralpha pathway in macrophages and its potential role in silica-induced pulmonary fibrosis. J Leukoc Biol. 2008. 83:630–639.

59. Mora AL, Torres-González E, Rojas M, Corredor C, Ritzenthaler J, Xu J, et al. Activation of alveolar macrophages via the alternative pathway in herpesvirus-induced lung fibrosis. Am J Respir Cell Mol Biol. 2006. 35:466–473.

60. Iida J, Une T, Ishihara C, Nishimura K, Tokura S, Mizukoshi N, et al. Stimulation of non-specific host resistance against Sendai virus and Escherichia coli infections by chitin derivatives in mice. Vaccine. 1987. 5:270–274.

61. Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci. 2008. 13:453–461.

62. Da Silva CA, Hartl D, Liu W, Lee CG, Elias JA. TLR-2 and IL-17A in chitin-induced macrophage activation and acute inflammation. J Immunol. 2008. 181:4279–4286.
63. Andersson LI, Hellman P, Eriksson H. Receptor-mediated endocytosis of particles by peripheral dendritic cells. Hum Immunol. 2008. 69:625–633.

64. van Vliet SJ, García-Vallejo JJ, van Kooyk Y. Dendritic cells and C-type lectin receptors: coupling innate to adaptive immune responses. Immunol Cell Biol. 2008. 86:580–587.

65. Andersen OA, Dixon MJ, Eggleston IM, van Aalten DM. Natural product family 18 chitinase inhibitors. Nat Prod Rep. 2005. 22:563–579.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download