Abstract
The invariant (i) natural killer (NK)T cells represent a unique subset of T lymphocytes which express the Vα14 chain of the T cell receptor (TCR), that recognizes glycolipid antigens presented by the nonpolymorphic major histocompatibility complex (MHC) class I-like antigen presentation molecule CD1d, and they participate in protection against some microbial pathogens. Although iNKT cells have originally been regarded as T cells co-expressing NKR-P1B/C (NK1.1: CD 161), they do not seem to consistently express this marker, since NK1.1 surface expression on iNKT cells undergoes dramatic changes following facultative intracellular bacterial infection, which is correlated with functional changes of this cell population. Accumulating evidence suggests that NK1.1 allows recognition of "missing-self", thus controling activation/inhibition of NK1.1-expressing cells. Therefore, it is tempting to suggest that iNKT cells participate in the regulation of host immune responses during facultative intracellular bacterial infection by controlling NK1.1 surface expression. These findings shed light not only on the unique role of iNKT cells in microbial infection, but also provide evidence for new aspects of the NK1.1 as a regulatory molecule on these cells.
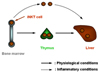
Facultative intracellular bacteria comprise various pathogens such as Mycobacterium tuberculosis, M. leprae, Salmonella enterica, Brucella sp., Legionella sp., Listeria monocytogenes, and Francisella tularensis, all of which can survive in professional phagocytes such as macrophages.1 Although innate immunity orchestrated by multiple cell populations, including granulocytes and macrophages, is pivotal for the elimination of these bacteria, conventional T cells are mandatory for sterile eradication of these pathogens (Fig. 1).1 Because the majority of facultative intracellular bacteria are trapped in the liver immediately after systemic infection, T cells that reside in and/or infiltrate the liver should play a decisive role in the following course of infection (Fig. 1). Experimental murine listeriosis models are instrumental in analyzing the role of T cells in the liver during facultative intracellular bacterial infection, not only because protection against L. monocytogenes strictly depends on T cells, but also because liver parenchymal cells serve as a reservoir for this bacterium (Fig. 1).1-3
The liver is a rich provenance of unconventional T cells, called natural killer (NK)T cells, co-expressing NKR-P1B/C (NK1.1)(CD161) that are type II membrane glycoproteins of the C-type lectin superfamily.4 The majority of NKT cells express an invariant (i) T cell receptor (TCR), typically comprising Vα14/Jα18 combined with a highly skewed TCRVβ towards Vβ8.2 in mouse, and homologous chain Vα24/Jα18 paired with Vβ11 in human (iNKT cells).4 The liver iNKT cells have a great potential to secrete both type 1 and type 2 cytokines.4-7 The high abundance of iNKT cells in the liver and their rapid and vigorous cytokine release in response to stimuli suggest participation of this cell population as an immunomodulator in the liver.
iNKT cells have been shown to participate in the regulation of various immune responses; e.g. tumor rejection8,9 and prevention of the development of autoimmune diseases.10-12 Although iNKT cells have been suggested to participate in elimination of various microbial pathogens,13-26 recent studies argue against the crucial role of this cell population in some microbial infections.27-32 Moreover, new studies have shed light on the intriguing aspects of the NKR-P1 family, including NKR-P1B/C (NK1.1), in controlling immune responses.33-40 Thus, iNKT cells appear to play more complicated roles than originally thought. Here, we focus on the unique aspects of iNKT cells as regulatory cells during murine listeriosis and the role of NK1.1 expressed on these cells.
Although iNKT cells were originally regarded as T cells co-expressing NK1.1, this cell population does not seem to consistently express this marker.41-44 Immature iNKT cells lack surface expression of NK1.1, but they acquire the marker expression during ontogeny, suggesting that the NK1.1- subset is a precursor of NK1.1+ subpopulation.43,44 Yet, substantial numbers of iNKT cells lacking NK1.1 have been identified in the periphery.28,41,42 This suggests that NK1.1 is not merely a marker for mature iNKT cells and raises the possibility that NK1.1 surface expression on iNKT cells is fluctuated under various conditions.
iNKT cells become undetectable upon activation.6,7,42,45-61 Although the disappearance of iNKT cells had been considered to be caused by activation-induced cell death/apoptosis (Fig. 2A),48,51,53,61 recent studies suggest that iNKT cells robustly expand in situ rather than undergoing apoptosis.57-59: i.e. the failure of iNKT cell detection is caused by the loss of NK1.1 and TCR, which were previously considered reliable markers for the detection of iNKT cells (Fig. 2A).57-59 Yet, the loss of surface expression of NK1.1 and TCR, and subsequent re-expression of marker(s) have thus far been observed only in iNKT cells stimulated with their agonist, α-galactoceramide (α-GalCer).57-59
Cells stained with monoclonal antibodies (mAbs) against surface markers, including NK1.1 and TCR, become transiently undetectable in the liver of mice following L. monocytogenes infection.6,46 This dynamic fluctuation of iNKT cells during L. monocytogenes infection has recently been verified using α-GalCer-loaded CD1d tetramers (α-GalCer/CD1d tetramers): the numbers of α-GalCer/CD1d tetramer-reactive T cells co-expressing NK1.1 (NK1.1+ iNKT cells) are markedly reduced in the liver during the early stages of L. monocytogenes infection, whereas those of α-GalCer/CD1d tetramer-reactive T cells lacking NK1.1 (NK 1.1- iNKT cells) rise following infection and become dominant among the iNKT cell population (Fig. 2B).28 Similar kinetics of iNKT cells are seen in Vβa mice, which are devoid of iNKT cells expressing TCRVβ8,28 implying that the curtailment of the NK1.1+ subset and subsequent expansion of NK 1.1- subpopulation occur independently from TCRVβ usage. Hence, iNKT cells are markedly influenced by L. monocytogenes infection, and the NK1.1+ and NK1.1- subsets of iNKT cells show differential kinetics during listeriosis.
Interleukin (IL)-12 secreted mainly from macrophages, dendritic cells, and granulocytes is a heterodimeric cytokine which is composed of a covalently linked 35-kDa light chain (p35) and a 40-kDa heavy chain (p40).62 IL-12 stimulates type 1 immune effector functions (e.g. promotion of IFNγ secretion from Th1 cells and NK cells),62 and hence, are mandatory for protection against intracellular microorganisms including L. monocytogenes.2,63
Neutralization of endogenous IL-12 (p40) reverses the curtailment of the NK1.1+ subset and the subsequent expansion of the NK1.1- subpopulation of iNKT cells during listeriosis.28,46 The kinetics of NK1.1+ and NK1.1- iNKT cells in the liver following L. monocytogenes infection is paralleled by numerical changes of IL-12 producers in the liver.28,64,65 It is thus conceivable that fluctuation of iNKT cells during listeriosis is determined by IL-12 (p40) levels in the hepatic microenvironment.
Although IL-12 (p40) had been considered a useful tool for determination of the role of IL-12 in vivo, the p40 subunit of IL-12 has been demonstrated to be shared by IL-23, a cytokine which is a disulfide-bridged complex of a p19 subunit and the p40 subunit of IL-12.62,66 It is therefore possible that IL-23 rather than IL-12 participates in the fluctuation of iNKT cells during L. monocytogenes infection. Yet, similarly to L. monocytogenes infection, numbers of NK1.1+ subset are markedly diminished following in vivo administration of recombinant (r)IL-12, and the NK1.1- subpopulation expands thereafter (Fig. 2B).28 Thus, it is tempting to assume that endogenous IL-12 plays a central role in the fluctuation of iNKT cells during listeriosis, although it cannot completely be excluded that IL-23 also, at least in part, participates in this mechanism.
iNKT cells become undetectable after stimulation with their agonist, α-GalCer.42,49,50,54,56-59 In contrast to L. monocytogenes infection, this change is not prevented by IL-12 neutralization (Fig. 2A).64 Thus, endogenous IL-12 is involved in the disappearance of NK1.1+ iNKT cells during L. monocytogenes infection (Fig. 2B),28,46 whereas the disappearance of NK1.1+ iNKT cells by α-GalCer treatment apparently occurs independently from IL-12 (Fig. 2A).64
The TCR surface expression on iNKT cells is down-modulated after α-GalCer stimulation (Fig. 2A),57-59 whereas that on iNKT cells is not affected by Salmonella infection.58 Similar to L. monocytogenes infection, fluctuation of iNKT cells during salmonellosis is also controlled by endogenous IL-12 [Emoto, unpublished observation]. It is therefore assumed that disappearance of NK1.1+ subset and subsequent expansion of NK1.1- subpopulation are differentially controlled by signalling though TCR and IL-12R.
The numerical increase of liver NK1.1- iNKT cells after L. monocytogenes infection is prevented by NK1.1+ cell depletion.28 This suggests that NK1.1+ cells are a prerequisite for the emergence of NK1.1- iNKT cells. Because NK1.1+ cells comprise not only iNKT cells, but also NK cells, it is possible that the numerical increase of NK1.1- iNKT cells following L. monocytogenes infection is controlled by NK cells. However, because numerical increase of NK1.1- iNKT cells following L. monocytogenes infection is further increased by NK cell depletion (anti-asialo GM1 Ab treatment),28 this possibility is unlikely.
In addition to NK and iNKT cells, NK1.1+ cells comprise nonclassical NKT cells. Although iNKT cells comprise cells expressing CD4 but lacking CD8 (CD4+ cells), and those lacking CD4 and CD8 (CD4-8- (double negative: DN) cells), a small but distinct NKT cell population expressing CD8 has been identified.67-71 Moreover, in contrast to iNKT cells which express TCRα/β as an antigen receptor, some NKT cells expressing TCRγ/δ have also been identified.70,72-75 Because both cell populations are also abundant in the liver70,75 and are depleted by NK1.1+ cell depletion, it is possible that the prevention of the emergence of NK1.1- iNKT cells by NK1.1+ cell depletion is caused by the depletion of these cells. However, because numerical increase of liver NK1.1- iNKT cells following L. monocytogenes infection is not prevented by CD8α+ or TCRγ/δ+ cell depletion,28 this possibility is also unlikely. Thus, the NK1.1- subset, which emerges in the liver of L. monocytogenes-infected mice, is primarily derived from the NK1.1+ subpopulation of iNKT cells.
Recent studies suggest that iNKT cells expand in situ robustly in response to α-GalCer.57-59 It is possible that a similar mechanism exists in listerial infection, because total numbers of iNKT cells are increased, though transiently reduced, following L. monocytogenes infection compared to preinfection.28 However, because (i) numbers of NK cells are increased in the liver following L. monocytogenes infection,27,28 (ii) expansion of iNKT cells is impaired in the presence of NK cells,76,77 and (iii) expansion of liver iNKT cells is promoted by NK cell depletion, which is caused by increased local concentrations of IL-1528,76 (IL-15 is prerequisite for the proliferation of not only NK cells but also iNKT cells78), it is conceivable that iNKT cells increased in the liver following L. monocytogenes infection are supplied from other organs. Hence, it is likely that stimulation by a specific antigen (i.e. α-GalCer) and by the cytokine IL-12 have differential outcomes.
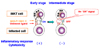
Although thymus is essential for the development of iNKT cells (Fig. 3),5,70,79-81 comparable numbers of those cells are detected in adult thymectomized mice.28,82 Similar to euthymic mice, numbers of the NK1.1+ subset are markedly diminished in adult thymectomized mice following L. monocytogenes infection, and the NK1.1- subpopulation is numerically increased.28 Thus, numerical alterations in NK1.1+ and NK1.1- iNKT cell populations in response to L. monocytogenes infection occur independently from a functional thymus. The NK1.1+ subset in the periphery has been found to be derived from a thymic NK1.1- subpopulation of iNKT cells.43,44 In this study, NK1.1 surface expression on iNKT cells is acquired in the periphery after the cells have left the thymus during ontogeny. However, the NK1.1- subset that emerges in the liver following L. monocytogenes infection develops from the NK1.1+ subpopulation of iNKT cells.28 Because the disappearance of liver iNKT cells after TCR stimulation has been shown to be followed by repopulation of these cells due to homeostatic proliferation of an iNKT-cell reservoir in the bone marrow,48 it is conceivable that some NK1.1+ iNKT cells, which re-emerge at later stages of listeriosis, are derived from the bone marrow, although in situ expansion of iNKT cells cannot completely be excluded (Fig. 3). Hence, accumulation of iNKT cells in the liver is differentially regulated under physiological and inflammatory conditions.48
Substantial numbers of IFN-γ and IL-4 producers are detected among liver iNKT cells from uninfected mice after in vitro stimulation with TCR/CD3 ligation5,6 or with phorbol myristate acetate and ionomycine28 (Fig. 4). High numbers of IFN-γ producers are detected among liver iNKT cells from L. monocytogenes-infected mice, whereas IL-4-producing cells are virtually undetectable (Fig. 4).6,28,46 Because the vast majority of iNKT cells in the liver of L. monocytogenes-infected mice lack surface expression of NK1.1, these findings suggest that NK1.1- iNKT cells, which emerge in the L. monocytogenes-infected liver, fail to produce IL-4 and hence express a Th1-like phenotype.
In mouse, the iNKT cells segregate into 2 populations on the basis of CD4 expression; i.e. CD4+ and DN cells.4,5,83 Before infection, the majority of liver iNKT cells co-express CD4 and only a minority lack this marker.5,28 The CD4+ iNKT cells are numerically reduced after L. monocytogenes infection, whereas DN iNKT cells are virtually unaffected (Fig. 4).6,28,46 Hence, L. monocytogenes infection primarily compresses the CD4+ rather than DN iNKT-cell population.
Since the CD4+ and DN iNKT cells are differentially influenced by L. monocytogenes infection, it is possible that each cell subset plays a different role during L. monocytogenes infection. In uninfected mice, frequencies of IL-4-producing iNKT cells are markedly reduced by CD4+ or NK1.1+ cell depletion, whereas numbers of IFN-γ producers are virtually unaffected.5,28 In contrast, frequencies of IFN-γ-producing iNKT cells are markedly higher in CD4+ cell-depleted mice after infection as compared to nondepleted mice.28 These findings suggest that NK1.1- iNKT cells with IFN-γ-producing activity in the L. monocytogenes-infected liver are preferentially DN (Fig. 4).
DN iNKT cells differ from CD4+ iNKT cells in their cytokine production profile.69,84-88 In general, CD4+ rather than DN iNKT cells are responsible for IL-4 production albeit at varying levels in different organs. Since (i) CD4+ cells dominate DN cells among liver iNKT cells,4,5,28 (ii) IL-4-producing cells in the liver are markedly reduced by NK1.1+ cell depletion,6,46 (iii) large numbers of IL-4 producers are detected among purified liver CD4+ NK1.1+ cells after TCR ligation,6 and (iv) numbers of IL-4-producing iNKT cells from CD4+ or NK1.1+ cell-depleted mice are minute,6,28 it appears that the CD4+NK1.1+ subset under physiological conditions is mainly responsible for IL-4 production in the liver although iNKT cells express both IL-4 and IFN-γ mRNA upon stimulation.89,90 Differential stimulation (i.e. specific antigen versus IL-12) is probably responsible for distinct cytokine production with inflammation, thus driving IFN-γ production from iNKT cells.5,28,46
Numbers of iNKT cells reach levels comparable to those in uninfected animals at later stages of listeriosis.46,64 In parallel, the NK1.1+ subset is proportionally increased and NK1.1- subpopulation is reduced. It is thus possible that NK1.1 is re-expressed on iNKT cells even if the marker was lost. Substantial numbers of donor-derived NK1.1+ iNKT cells are detected in the liver of recipient re-arrangement gene-1-/- mice lacking all T cells, including iNKT cells, which was reconstituted with NK1.1+ cell-depleted hepatic leukocytes from mice that had been infected with L. monocytogenes.28 These findings suggest that NK1.1 can be re-expressed on iNKT cells, even in spite of previous loss of the marker (Fig. 2B), although it cannot completely be excluded that NK1.1- iNKT cells fail to accumulate in the liver of recipient mice. It is conceivable that at least NK1.1+ iNKT cells, which re-emerge during late stages of listeriosis, express functional activities similar to those in naive mice, because NK1.1+ iNKT cells that re-emerged in the liver have a potential to secrete IL-4.64
Re-expression of NK1.1 on liver iNKT cells from mice, which had been infected with L. monocytogenes, is not found after in-vitro culture even in the presence of IL-12-neutralizing mAb [Emoto et al., unpublished observation]. This is consistent with previous findings showing that TCR, but not NK1.1, becomes detectable on α-GalCer-stimulated iNKT cells after in-vitro culture.58 These findings suggest that different mechanisms exist in the disappearance/re-emergence of NK1.1 and TCR, and that some factor(s) other than IL-12 also participate in down-modulation of NK1.1 on iNKT cells.
The NKR-P1 family comprises activatory and inhibitory receptors. Whereas NKR-P1A, NKR-P1C, and NKR-P1F are activatory receptors, NKR-P1B and NKR-P1D are inhibitory receptors.33-40,91 Lectin-like transcript 1 or C-type lectin-related molecules have been identified as ligands for NKR-P1A, NKR-P1B, NKR-P1D, and NKR-P1F.38-40 Thus, NKR-P1 family members allow recognition of "missing-self", thus controlling activation/inhibition of NK1.1+ cells in a MHC class I-independent manner. Because cross-linking of NKR-P1C by anti-NK1.1 mAb induces IFN-γ production from NK1.1+ cells,33 it is possible that NKR-P1C participates in immunosurveillance such as the elimination of cells lacking hither-to-unknown antigen(s) expressed on infected cells (Fig. 5). It is therefore possible that NK1.1 participates in surveillance of infection, and that the loss of NK1.1 counteracts excessive inflammatory responses.
IFN-γ plays an essential role in resistance against L. monocytogenes infection,2,92-95 whereas IL-4 exacerbates disease.96-99 Since considerable numbers of IFN-γ, but not IL-4-secreting iNKT cells, are found in the liver of L. monocytogenes-infected mice, it is possible that iNKT cells participate in protection against L. monocytogenes infection. Yet, Jα18-/- mice, which are entirely devoid of iNKT cells, are more resistant to L. monocytogenes infection than control mice.28 These findings suggest that iNKT cells do not participate in antilisterial resistance and may even exacerbate disease, although contribution of iNKT cells in protection against enteric listeriosis has been suggested.100 Because iNKT cells comprise a heterogeneous population, it is speculated that the IFN-γ-producing NK1.1- subset of iNKT cells ameliorates, whereas the IL4-producing NK1.1+ subpopulation exacerbates disease (see Fig. 4).
At first sight, the finding that listeriosis in mice lacking total iNKT cells is ameliorated could be taken as an argument against a pivotal role of the iNKT cells in protection against L. monocytogenes infection. However, 2 subsets of iNKT cells exist: (i) The CD4+NK1.1+ subset which produces IL-4 and hence should be of detriment in listeriosis. At early stages of infection, exacerbation by this subset seems to dominate because depletion of the total iNKT cell population ameliorates listeriosis. This notion is consistent with previous findings showing that listeriosis is improved by anti-CD1 mAb treatment.29 (ii) The CD4-NK1.1- subset produces IFN-γ suggesting its beneficial role in listeriosis. Yet, contribution of the NK1.1- subset to resistance occurs later and seems supportive but not essential.
NK1.1 surface expression and functional activities of iNKT cells are markedly influenced by listerial infection. Despite the designation of NKT cells, the NK1.1 surface molecule is not a reliable marker of this cell population. Although iNKT cells produce both IFN-γ and IL-4 in naive mice, the majority of this cell population produces IFN-γ during listeriosis, but not IL-4 due to abundant IL-12 in microenvironment. It is therefore tempting to assume that distinct iNKT-cell populations play different roles in intracellular bacterial infection. Of these, the NK1.1+ subset seems ineffectual or even harmful, whereas the NK1.1- subset appears to contribute to antilisterial protection by means of IFN-γ. NK1.1 surface expression on iNKT cells in the liver fluctuates during L. monocytogenes infection in a reversible manner. This dynamic fluctuation of NK1.1 expression on iNKT cells suggests a unique role of the NK1.1 molecule on this cell population during intracellular bacterial infection.
Figures and Tables
Notes
This work was supported by a Grant-in-Aid for Scientific Research (17590383) from the Japan Society for the Promotion of Science, The Waksman Foundation of Japan Inc., The Japan Research Foundation for Clinical Pharmacology, Mishima Kaiun Memorial Foundation, Hokuto Foundation for Bioscience, and the German Science Foundation (SFB 421).
References
1. Kaufmann SH. Paul WE, editor. Immunity to intracellular bacteria. Fundamental Immunology. 2003. 5th ed. Philadelphia: Lippincott-Raven Publishers;1229–1261.
2. North RJ, Conlan JW. Immunity to Listeria monocytogenes. Chem Immunol. 1998. 70:1–20.
3. Gregory SH, Wing EJ. Neutrophil-Kupffer-cell interaction in host defenses to systemic infections. Immunol Today. 1998. 19:507–510.

4. Emoto M, Kaufmann SH. Liver NKT cells: an account of heterogeneity. Trends Immunol. 2003. 24:364–369.

5. Emoto M, Emoto Y, Kaufmann SH. IL-4 producing CD4+ TCRαβint liver lymphocytes: influence of thymus, β2-microglobulin and NK1.1 expression. Int Immunol. 1995. 7:1729–1739.

6. Emoto M, Emoto Y, Kaufmann SH. Interleukin-4-producing CD4+ NK1.1+ TCRαβintermediate liver lymphocytes are down-regulated by Listeria monocytogenes. Eur J Immunol. 1995. 25:3321–3325.

7. Emoto M, Emoto Y, Buchwalow IB, Kaufmann SH. Induction of IFN-γ-producing CD4+ natural killer T cells by Mycobacterium bovis bacillus Calmette Guérin. Eur J Immunol. 1999. 29:650–659.

8. Cui J, Shin T, Kawano T, Sato H, Kondo E, Toura I, et al. Requirement for Vα14 NKT cells in IL-12-mediated rejection of tumors. Science. 1997. 278:1623–1626.

9. Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Sato H, et al. Natural killer-like nonspecific tumor cell lysis mediated by specific ligand-activated Vα14 NKT cells. Proc Natl Acad Sci U S A. 1998. 95:5690–5693.

10. Falcone M, Yeung B, Tucker L, Rodriguez E, Sarvetnick N. A defect in interleukin 12-induced activation and interferon γ secretion of peripheral natural killer T cells in nonobese diabetic mice suggests new pathogenic mechanisms for insulin-dependent diabetes mellitus. J Exp Med. 1999. 190:963–972.

11. Miyamoto K, Miyake S, Yamamura T. A synthetic glycolipid prevents autoimmune encephalomyelitis by inducing TH2 bias of natural killer T cells. Nature. 2001. 413:531–534.

12. Hong S, Wilson MT, Serizawa I, Wu L, Singh N, Naidenko OV, et al. The natural killer T-cell ligand α-galactosylceramide prevents autoimmune diabetes in non-obese diabetic mice. Nat Med. 2001. 7:1052–1056.

13. Denkers EY, Scharton-Kerston T, Barbieri S, Caspar P, Sher A. A role for CD4+NK1.1+ T lymphocytes as major histocompatibility complex class II independent helper cells in the generation of CD8+ effector function against intracellular infection. J Exp Med. 1996. 184:131–139.

14. Ishikawa H, Hisaeda H, Taniguchi M, Nakayama T, Sakai T, Maekawa Y, et al. CD4+ Vα14 NKT cells play a crucial role in an early stage of protective immunity against infection with Leishmania major. Int Immunol. 2000. 12:1267–1274.

15. Gonzalez-Aseguinolaza G, de Oliveira C, Tomaska M, Hong S, Bruna-Romero O, Nakayama T, et al. α-galactosylceramide-activated Vα14 natural killer T cells mediate protection against murine malaria. Proc Natl Acad Sci U S A. 2000. 97:8461–8466.

16. Kawakami K, Kinjo Y, Yara S, Koguchi Y, Uezu K, Nakayama T, et al. Activation of Vα14+ natural killer T cells by α-galactosylceramide results in development of Th1 response and local host resistance in mice infected with Cryptococcus neoformans. Infect Immun. 2001. 69:213–220.

17. Exley MA, Bigley NJ, Cheng O, Tahir SM, Smiley ST, Carter QL, et al. CD1d-reactive T-cell activation leads to amelioration of disease caused by diabetogenic encephalomyocarditis virus. J Leukoc Biol. 2001. 69:713–718.
18. Duthie MS, Wleklinski-Lee M, Smith S, Nakayama T, Taniguchi M, Kahn SJ. During Trypanosoma cruzi infection CD1-restricted NK T cells limit parasitemia and augment the antibody response to a glycophosphoinositol-modified surface protein. Infect Immun. 2002. 70:36–48.

19. Johnson TR, Hong S, van Kaer L, Koezuka Y, Graham BS. NK T cells contribute to expansion of CD8+ T cells and amplification of antiviral immune responses to respiratory syncytial virus. J Virol. 2002. 76:4294–4303.

20. Nieuwenhuis EE, Matsumoto T, Exley M, Schleipman RA, Glickman J, Bailey DT, et al. CD1d-dependent macrophage-mediated clearance of Pseudomonas aeruginosa from lung. Nat Med. 2002. 8:588–593.
21. Brigl M, Bry L, Kent SC, Gumperz JE, Brenner MB. Mechanism of CD1d-restricted natural killer T cell activation during microbial infection. Nat Immunol. 2003. 4:1230–1237.

22. Grubor-Bauk B, Simmons A, Mayrhofer G, Speck PG. Impaired clearance of herpes simplex virus type 1 from mice lacking CD1d or NKT cells expressing the semivariant Vα14-Jα281 TCR. J Immunol. 2003. 170:1430–1434.

23. Kawakami K, Yamamoto N, Kinjo Y, Miyagi K, Nakasone C, Uezu K, et al. Critical role of Vα14+ natural killer T cells in the innate phase of host protection against Streptococcus pneumoniae infection. Eur J Immunol. 2003. 33:3322–3330.
24. Amprey JL, Im JS, Turco SJ, Murray HW, Illarionov PA, Besra GS, et al. A subset of liver NK T cells is activated during Leishmania donovani infection by CD1d-bound lipophosphoglycan. J Exp Med. 2004. 200:895–904.

25. Smiley ST, Lanthier PA, Couper KN, Szaba FM, Boyson JE, Chen W, et al. Exacerbated susceptibility to infection-stimulated immunopathology in CD1d-deficient mice. J Immunol. 2005. 174:7904–7911.
26. Behar SM, Dascher CC, Grusby MJ, Wang CR, Brenner MB. Susceptibility of mice deficient in CD1D or TAP1 to infection with Mycobacterium tuberculosis. J Exp Med. 1999. 189:1973–1980.
27. Teixeira HC, Kaufmann SH. Role of NK1.1+ cells in experimental listeriosis. NK1+ cells are early IFN-γ producers but impair resistance to Listeria monocytogenes infection. J Immunol. 1994. 152:1873–1882.
28. Emoto M, Yoshizawa I, Emoto Y, Miamoto M, Hurwitz R, Kaufmann SH. Rapid development of a gamma interferon-secreting glycolipid/CD1d-specific Vα14+NK 1.1- T-cell subset after bacterial infection. Infect Immun. 2006. 74:5903–5913.

29. Szalay G, Ladel CH, Blum C, Brossay L, Kronenberg M, Kaufmann SH. Cutting edge: anti-CD1 monoclonal antibody treatment reverses the production patterns of TGF-β2 and Th1 cytokines and ameliorates listeriosis in mice. J Immunol. 1999. 162:6955–6958.
30. Kawakami K, Kinjo Y, Uezu K, Yara S, Miyagi K, Koguchi Y, et al. Minimal contribution of Vα14 natural killer T cells to Th1 response and host resistance against mycobacterial infection in mice. Microbiol Immunol. 2002. 46:207–210.

31. Bilenki L, Wang S, Yang J, Fan Y, Joyee AG, Yang X. NK T cell activation promotes Chlamydia trachomatis infection in vivo. J Immunol. 2005. 175:3197–3206.

32. Cornish AL, Keating R, Kyparissoudis K, Smyth MJ, Carbone FR, Godfrey DI. NKT cells are not critical for HSV-1 disease resolution. Immunol Cell Biol. 2006. 84:13–19.

33. Arase H, Arase N, Saito T. Interferon γ production by natural killer (NK) cells and NK1.1+ T cells upon NKR-P1 cross-linking. J Exp Med. 1996. 183:2391–2396.

34. Arase N, Arase H, Park SY, Ohno H, Ra C, Saito T. Association with FcRγ is essential for activation signal through NKR-P1 (CD161) in natural killer (NK) cells and NK1.1+ T cells. J Exp Med. 1997. 186:1957–1963.
35. Ryan JC, Seaman WE. Divergent functions of lectin-like receptors on NK cells. Immunol Rev. 1997. 155:79–89.

36. Carlyle JR, Martin A, Mehra A, Attisano L, Tsui FW, Zúñiga-Pflücker JC. Mouse NKR-P1B, a novel NK1.1 antigen with inhibitory function. J Immunol. 1999. 162:5917–5923.
37. Kung SK, Su RC, Shannon J, Miller RG. The NKR-P1B gene product is an inhibitory receptor on SJL/J NK cells. J Immunol. 1999. 162:5876–5887.
38. Iizuka K, Naidenko OV, Plougastel BF, Fremont DH, Yokoyama WM. Genetically linked C-type lectin-related ligands for the NKR-P1 family of natural killer cell receptors. Nat Immunol. 2003. 4:801–807.

39. Carlyle JR, Jamieson AM, Gasser S, Clingan CS, Arase H, Raulet DH. Missing self-recognition of Ocil/Clr-b by inhibitory NKR-P1 natural killer cell receptors. Proc Natl Acad Sci U S A. 2004. 101:3527–3532.

40. Aldemir H, Prod'homme V, Dumaurier MJ, Retiere C, Poupon G, Cazareth J, et al. Cutting edge: lectin-like transcript 1 is a ligand for the CD161 receptor. J Immunol. 2005. 175:7791–7795.
41. Benlagha K, Weiss A, Beavis A, Teyton L, Bendelac A. In vivo identification of glycolipid antigen-specific T cells using fluorescent CD1d tetramers. J Exp Med. 2000. 191:1895–1903.
42. Matsuda JL, Naidenko OV, Gapin L, Nakayama T, Taniguchi M, Wang CR, et al. Tracking the response of natural killer T cells to a glycolipid antigen using CD1d tetramers. J Exp Med. 2000. 192:741–754.
43. Pellicci DG, Hammond KJ, Uldrich AP, Baxter AG, Smyth MJ, Godfrey DI. A Natural killer T (NKT) cell developmental pathway involving a thymus-dependent NK1.1-CD4+ CD1d-dependent precursor stage. J Exp Med. 2002. 195:835–844.
44. Benlagha K, Kyin T, Beavis A, Teyton L, Bendelac A. A thymic precursor to the NK T cell lineage. Science. 2002. 296:553–555.

45. Emoto M, Emoto Y, Kaufmann SH. Bacille Calmette Guérin and interleukin-12 down-modulate interleukin-4-producing CD4+NK1+ T lymphocytes. Eur J Immunol. 1997. 27:183–188.

46. Emoto Y, Emoto M, Kaufmann SH. Transient control of interleukin-4-producing natural killer T cells in the livers of Listeria monocytogenes-infected mice by interleukin-12. Infect Immun. 1997. 65:5003–5009.
47. Chen H, Huang H, Paul WE. NK1.1+ CD4+ T cells lose NK11 expression upon in vitro activation. J Immunol. 1997. 158:5112–5119.
48. Eberl G, MacDonald HR. Rapid death and regeneration of NKT cells in anti-CD3ε- or IL-12-treated mice: a major role for bone marrow in NKT cell homeostasis. Immunity. 1998. 9:345–353.

49. Eberl G, MacDonald HR. Selective induction of NK cell proliferation and cytotoxicity by activated NKT cells. Eur J Immunol. 2000. 30:985–992.
50. Osman Y, Kawamura T, Naito T, Takeda K, van Kaer L, Okumura K, et al. Activation of hepatic NKT cells and subsequent liver injury following administration of α-galactosylceramide. Eur J Immunol. 2000. 30:1919–1928.

51. Leite-de-Moraes MC, Herbelin A, Gouarin C, Koezuka Y, Schneider E, Dy M. Fas/Fas ligand interactions promote activation-induced cell death of NK T lymphocytes. J Immunol. 2000. 165:4367–4371.

52. Daniels KA, Devora G, Lai WC, O'Donnell CL, Bennett M, Welsh RM. Murine cytomegalovirus is regulated by a discrete subset of natural killer cells reactive with monoclonal antibody to Ly49H. J Exp Med. 2001. 194:29–44.

53. Hobbs JA, Cho S, Roberts TJ, Sriram V, Zhang J, Xu M, et al. Selective loss of natural killer T cells by apoptosis following infection with lymphocytic choriomeningitis virus. J Virol. 2001. 75:10746–10754.

54. Fujii S, Shimizu K, Kronenberg M, Steinman RM. Prolonged IFN-γ-producing NKT response induced with α-galactosylceramide-loaded DCs. Nat Immunol. 2002. 3:867–874.

55. Kirby AC, Yrlid U, Wick MJ. The innate immune response differs in primary and secondary Salmonella infection. J Immunol. 2002. 169:4450–4459.

56. Motsinger A, Haas DW, Stanic AK, van Kaer L, Joyce S, Unutmaz D. CD1d-restricted human natural killer T cells are highly susceptible to human immunodeficiency virus 1 infection. J Exp Med. 2002. 195:869–879.

57. Crowe NY, Uldrich AP, Kyparissoudis K, Hammond KJ, Hayakawa Y, Sidobre S, et al. Glycolipid antigen drives rapid expansion and sustained cytokine production by NK T cells. J Immunol. 2003. 171:4020–4027.

58. Wilson MT, Johansson C, Olivares-Villagómez D, Singh AK, Stanic AK, Wang C, et al. The response of natural killer T cells to glycolipid antigens is characterized by surface receptor down-modulation and expansion. Proc Natl Acad Sci U S A. 2003. 100:10913–10918.

59. Harada M, Seino K, Wakao H, Sakata S, Ishizuka Y, Ito T, et al. Down-regulation of the invariant Vα14 antigen receptor in NKT cells upon activation. Int Immunol. 2004. 16:241–247.

60. Berntman E, Rolf J, Johansson C, Anderson P, Cardell SL. The role of CD1d-restricted NK T lymphocytes in the immune response to oral infection with Salmonella typhimurium. Eur J Immunol. 2005. 35:2100–2109.

61. Lin Y, Roberts TJ, Wang CR, Cho S, Brutkiewicz RR. Long-term loss of canonical NKT cells following an acute virus infection. Eur J Immunol. 2005. 35:879–889.

62. Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003. 3:133–146.

63. Tripp CS, Gately MK, Hakimi J, Ling P, Unanue ER. Neutralization of IL-12 decreases resistance to Listeria in SCID and C.B-17 mice. Reversal by IFN-γ. J Immunol. 1994. 152:1883–1887.
64. Emoto M, Yoshizawa I, Emoto Y, Takahashi Y, Hurwitz R, Miamoto M, et al. Reversible NK1.1 surface expression by invariant liver natural killer T cells during Listeria monocytogenes infection. Microbes Infect. 2007. 9:1511–1520.

65. Emoto Y, Yoshizawa I, Hurwitz R, Brinkmann V, Kaufmann SH, Emoto M. Role of interleukin-12 in determining differential kinetics of invariant natural killer T cells in response to differential burden of Listeria monocytogenes. Microbes Infect. 2008. 10:224–232.

66. Oppmann B, Lesley R, Blom B, Timans JC, Xu Y, Hunte B, et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000. 13:715–725.

67. Eberl G, Lees R, Smiley ST, Taniguchi M, Grusby MJ, MacDonald HR. Tissue-specific segregation of CD1d-dependent and CD1d-independent NK T cells. J Immunol. 1999. 162:6410–6419.
68. Legendre V, Boyer C, Guerder S, Arnold B, Hämmerling G, Schmitt-Verhulst AM. Selection of phenotypically distinct NK1.1+ T cells upon antigen expression in the thymus or in the liver. Eur J Immunol. 1999. 29:2330–2343.

69. Hammond KJ, Pelikan SB, Crowe NY, Randle-Barrett E, Nakayama T, Taniguchi M, et al. NKT cells are phenotypically and functionally diverse. Eur J Immunol. 1999. 29:3768–3781.

70. Emoto M, Zerrahn J, Miyamoto M, Pérarnau B, Kaufmann SH. Phenotypic characterization of CD8+NKT cells. Eur J Immunol. 2000. 30:2300–2311.
71. Coles MC, McMahon CW, Takizawa H, Raulet DH. Memory CD8 T lymphocytes express inhibitory MHC-specific Ly49 receptors. Eur J Immunol. 2000. 30:236–244.

72. Koyasu S. CD3+CD16+NK1.1+B220+ large granular lymphocytes arise from both α-βTCR+CD4-CD8- and γ-δ TCR+CD4-CD8-cells. J Exp Med. 1994. 179:1957–1972.
73. Arase H, Ono S, Arase N, Park SY, Wakizaka K, Watanabe H, et al. Developmental arrest of NK1.1+ T cell antigen receptor (TCR)-α/β+ T cells and expansion of NK1.1+ TCR-γ/δ+ T cell development in CD3ζ-deficient mice. J Exp Med. 1995. 182:891–895.

74. Vicari AP, Mocci S, Openshaw P, O'Garra A, Zlotnik A. Mouse γδ TCR+NK1.1+ thymocytes specifically produce interleukin-4, are major histocompatibility complex class I independent, and are developmentally related to αβ TCR+NK1.1+ thymocytes. Eur J Immunol. 1996. 26:1424–1429.

75. Emoto M, Miyamoto M, Emoto Y, Zerrahn J, Kaufmann SH. A critical role of T-cell receptor γ/δ cells in antibacterial protection in mice early in life. Hepatology. 2001. 33:887–893.

76. Matsuda JL, Gapin L, Sidobre S, Kieper WC, Tan JT, Ceredig R, et al. Homeostasis of Vα14i NKT cells. Nat Immunol. 2002. 3:966–974.

77. Ranson T, Vosshenrich CA, Corcuff E, Richard O, Laloux V, Lehuen A, et al. IL-15 availability conditions homeostasis of peripheral natural killer T cells. Proc Natl Acad Sci U S A. 2003. 100:2663–2668.

78. Ohteki T, Ho S, Suzuki H, Mak TW, Ohashi PS. Role for IL-15/IL-15 receptor β-chain in natural killer 1.1+ T cell receptor-αβ+ cell development. J Immunol. 1997. 159:5931–5935.
79. Bendelac A. Positive selection of mouse NK1+ T cells by CD1-expressing cortical thymocytes. J Exp Med. 1995. 182:2091–2096.

80. Hammond K, Cain W, van Driel I, Godfrey D. Three day neonatal thymectomy selectively depletes NK1.1+ T cells. Int Immunol. 1998. 10:1491–1499.
81. Tilloy F, Di Santo JP, Bendelac A, Lantz O. Thymic dependence of invariant Vα14+ natural killer-T cell development. Eur J Immunol. 1999. 29:3313–3318.
82. Kameyama H, Kawamura T, Naito T, Bannai M, Shimamura K, Hatakeyama K, et al. Size of the population of CD4+ natural killer T cells in the liver is maintained without supply by the thymus during adult life. Immunology. 2001. 104:135–141.

83. Bendelac A, Rivera MN, Park SH, Roark JH. Mouse CD1-specific NK1 T cells: development, specificity, and function. Annu Rev Immunol. 1997. 15:535–562.

84. Davodeau F, Peyrat MA, Necker A, Dominici R, Blanchard F, Leget C, et al. Close phenotypic and functional similarities between human and murine αβ T cells expressing invariant TCR α-chains. J Immunol. 1997. 158:5603–5611.
85. Hameg A, Apostolou I, Leite-De-Moraes M, Gombert JM, Garcia C, Koezuka Y, et al. A subset of NKT cells that lacks the NK1.1 marker, expresses CD1d molecules, and autopresents the α-galactosylceramide antigen. J Immunol. 2000. 165:4917–4926.

86. Takahashi T, Nieda M, Koezuka Y, Nicol A, Porcelli SA, Ishikawa Y, et al. Analysis of human Vα24+CD4+ NKT cells activated by α-glycosylceramide-pulsed monocyte-derived dendritic cells. J Immunol. 2000. 164:4458–4464.
87. Gumperz JE, Miyake S, Yamamura T, Brenner MB. Functionally distinct subsets of CD1d-restricted natural killer T cells revealed by CD1d tetramer staining. J Exp Med. 2002. 195:625–636.

88. Lee PT, Benlagha K, Teyton L, Bendelac A. Distinct functional lineages of human Vα24 natural killer T cells. J Exp Med. 2002. 195:637–641.

89. Matsuda JL, Gapin L, Baron JL, Sidobre S, Stetson DB, Mohrs M, et al. Mouse Vα14i natural killer T cells are resistant to cytokine polarization in vivo. Proc Natl Acad Sci U S A. 2003. 100:8395–8400.

90. Stetson DB, Mohrs M, Reinhardt RL, Baron JL, Wang ZE, Gapin L, et al. Constitutive cytokine mRNAs mark natural killer (NK) and NK T cells poised for rapid effector function. J Exp Med. 2003. 198:1069–1076.

91. Plougastel B, Matsumoto K, Dubbelde C, Yokoyama WM. Analysis of a 1-Mb BAC contig overlapping the mouse Nkrp1 cluster of genes: cloning of three new Nkrp1 members, Nkrp1d, Nkrp1e, and Nkrp1f. Immunogenetics. 2001. 53:592–598.

92. Holmberg LA, Ault KA. Characterization of Listeria monocytogenes-induced murine natural killer cells. Immunol Res. 1986. 5:50–60.

93. Bancroft GJ, Schreiber RD, Unanue ER. Natural immunity: a T-cell-independent pathway of macrophage activation, defined in the scid mouse. Immunol Rev. 1991. 124:5–24.

94. Huang S, Hendriks W, Althage A, Hemmi S, Bluethmann H, Kamijo R, et al. Immune response in mice that lack the interferon-γ receptor. Science. 1993. 259:1742–1745.

95. Harty JT, Bevan MJ. Specific immunity to Listeria monocytogenes in the absence of IFN γ. Immunity. 1995. 3:109–117.

96. Iizawa Y, Czuprynski C. Effects of administration of murine recombinant IL-4 on the resistance of mice to Listeria monocytogenes infection. Immunol Lett. 1992. 32:185–189.

97. Haak-Frendscho M, Brown JF, Iizawa Y, Wagner RD, Czuprynski CJ. Administration of anti-IL-4 monoclonal antibody 11B11 increases the resistance of mice to Listeria monocytogenes infection. J Immunol. 1992. 148:3978–3985.
98. Szalay G, Ladel CH, Blum C, Kaufmann SH. IL-4 neutralization or TNF-γ treatment ameliorate disease by an intracellular pathogen in IFN-γ receptor-deficient mice. J Immunol. 1996. 157:4746–4750.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download