INTRODUCTION
It is known that actinic keratosis and Bowen's disease, both intraepidermal skin tumors, have a potential progression to squamous cell carcinoma. Skin cancer constitutes one of the most frequent types of malignancies in humans with rapidly increasing worldwide incidences. Ultraviolet radiation (UVR) is an essential risk factor for the development of premalignant as well as malignant skin lesions.1 UVR can function as a complete carcinogen by inducing DNA mutations and by suppressing protective cellular antitumoral immune responses.2 Recent data showed that UVB-dendritic cell (DC) induces regulatory CD4+CD25+ cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4+) T cells.3
Dendritic cells (DCs) induce cutaneous immune response, however, several studies have suggested that DCs are involved in immunosuppression.4,5 DC-derived immunosuppression can result from antigen presentation by immature DCs that lack sufficient accessory molecules. In addition, immature DCs can differentiate into tolerogenic DCs under the influence of IL-10, TNFα and T regulatory cells (Tregs).5 Recently, epidermal expression of CD254 (receptor activator of NF-kappaB ligand, RANKL) has been shown to connect UVR with immunosuppression and tolerogenic phenotype in DCs and expansion of Tregs.6,7 Tregs are thought to be functionally unique population of T cells, and function to maintain immune homeostasis.8-10 Tregs have a functionally immunosuppressive property that inhibits effector cells to act against self in autoimmune diseases or a tumor. Foxp3, a member of the forkhead or winged helix family of transcription factors, is thought to be most reliable marker for Tregs.11,12 Foxp3 protein is responsible for scurfy mouse and human disorder of immune dysregulation, polyendocrinopathy, enteropathy, and X-linked inheritance, both of which are characterized by lack of CD4+CD25+ Tregs.13,14 Tregs may be highly relevant in cancer progression. Elevated numbers of Tregs have been found in cancer patients, and it has been shown that the elimination of Tregs may be used to enhance antitumoral immune responses.15-20 There is, however, limited information describing the prevalence of Tregs infiltration in premalignant and malignant skin lesions. The aim of this study was to evaluate the prevalence of Tregs and DCs infiltration in cutaneous premalignant and malignant squamous lesions by immunohis-tchemistry.
MATERIALS AND METHODS
Patients
Eighty-three patients with actinic keratosis, Bowen's disease or squamous cell carcinoma treated at Dongguk University Kyongju Hospital were enrolled in this study. The characteristics of the study subjects are summarized in Table 1. None of the patients received radiotherapy or chemotherapy before diagnosis.
Immunohistochemistry
Skin sections of 4µm thickness were made and spread on poly-L-lysine coated slides. Paraffin sections were immersed in three changes of xylene and hydrated using a graded series of alcohol. Antigen retrieval was performed routinely by immersing the sections in sodium citrate buffer (pH 6.0 for Ki67) or Tris-EDTA buffer (pH 6.0 for Foxp3) in a pressure cooker by autoclaving for 15 minutes. Endogenous peroxidase activity was blocked with 3% hydrogen peroxide for 15 minutes and then incubated with primary antibody for 2 hours at room temperature. Primary antibodies were rabbit polyclonal anti-S100 (DakoCytomation, Carpinteria, CA, USA, dilution 1 : 500), mouse monoclonal anti-Ki67 antibody (Zymed Laboratories, South San Franciso, CA, USA, dilution 1 : 200) and mouse monoclonal anti-Foxp3 antibody (Abcam, Cambridge, UK, dilution 1 : 100). Staining was done with a Dako EnVision kit labeled with peroxidase (DakoCytomation) and developed with 3, 3'-diaminobenzidine tetrahydrochloride (Zymed) as a chromogen. Sections were counterstained for 3 minutes with Meyers hematoxylin and then mounted. As a negative control, rabbit and mouse IgG isotypes were used instead of primary antibody. In addition, double-staining immunohistochemistry was performed in human tonsil with anti-Foxp3 antibody and anti-CD4 antibody (DakoCytomation) to confirm that Foxp3(+) cells were really CD4(+). Second staining was performed with a LSAB+kit labeled with alkaline phosphatase (DakoCytomation) and developed with Permanent Red (DakoCytomation) as a chromogen. The Foxp3 labeling index (LI) was calculated by counting positive cells in 500 lymphocytes throughout entire area of tumor stroma at a magnification of ×400. In the case of Ki67 LI, at least 500 malignant squamous cells were counted throughout entire area of tumor at a magnification of ×400. The number of S100 positive DCs in five randomly selected tumor stroma areas was analyzed at a magnification of ×400 and averaged. To examine the relationship of topographic distribution between S100 positive DCs and Tregs, figures of immunohistochemical staining for S100 and Foxp3 were merged on the Adobe Photoshop 9.0.
RESULTS
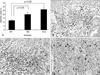
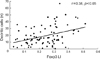
To confirm that Foxp3(+) cells were really CD4(+), double staining immunohistochemistry was done in human tonsil. Most of Foxp3(+) and CD4(+) lymphocytes were present in paracortical area. Some of CD4(+) lymphocytes showed positive signal for Foxp3 (Fig. 1A). Tregs stayed in close proximity to S100(+) DCs (Fig. 1B). Foxp3 LI was 0.28 ± 0.11, 0.27 ± 0.13 and 0.19 ± 0.08 in squamous cell carcinoma, Bowen's disease and actinic keratosis, respectively. As shown in Fig. 2. Foxp3 LI was significantly higher in squamous cell carcinoma and Bowen's disease than in actinic keratosis (p< 0.05). In each disease, there was no significant difference in Foxp3 LI between age and gender (data not shown). In squamous cell carcinoma, Foxp3 LI was 0.28 ± 0.13 and 0.30 ± 0.07 in well differentiation tumors (N = 13) and moderate to poor tumors (N = 14), respectively, and insignificantly related to Ki67 LI. There was also no significant difference in Foxp3 LI between tumor differentiation and Ki67 LI (p> 0.05). The number of S100 positive DCs was 26.45 ± 16.02, 20.21 ± 13.94 and 12.47 ± 7.03 in squamous cell carcinoma, Bowen's disease and actinic keratosis, respectively, As shown in Fig. 3, total DCs infiltration was significantly higher in squamous cell carcinoma and Bowen's disease than in actinic keratosis (p< 0.05). In each disease, there was no significant difference in the number of DCs between age and gender (data not shown). In squamous cell carcinoma, the number of DCs was 22.39 ± 11.76 and 29.60 ± 17.35 in well differentiation tumors (N= 13) and moderate to poor tumors (N = 14), respectively, and insignificantly related to Ki67 LI. There was also no significant difference in the number of DCs between tumor differentiation and Ki67 LI. As shown in Fig. 4, the number of DCs was closely correlated with Foxp3 LI (r = 0.378, p< 0.05).
DISCUSSION
For the first time, this study performed in situ analysis of Tregs in cutaneous premalignant and malignant squamous lesions, and showed that the population of Tregs and DCs were increased in Bowen's disease and cutaneous squamous cell carcinoma compared to actinic keratosis. In addition, Tregs infiltration was closely related with the number of infiltrating DCs, and Tregs were also located in direct proximity to DCs.
Naturally arising CD4+CD25+ Tregs characteristically express CD25, CTLA-4, glucocorticoid-induced tumor necrosis factor receptor family related gene (GITR), surface transforming growth factor-β (TGF-β), and Foxp3. CD25 is a critical molecule for proliferation and survival of CD4+CD25+ Tregs.21 However, CD25 is not a suitable marker to define Tregs because activated T cells generally express CD25. Compelling studies have revealed that CTLA-4 and TGF-β play roles in the suppressive activity of CD4+CD25+ Tregs against CD4+ or CD8+ T cells, although they are not expressed exclusively in Tregs. Experiments with Foxp3-overexpressing transgenic or Foxp3 gene-depleted mice and other studies have shown that Foxp3 is a master control gene for the development and function of natural CD4+CD25+ Tregs.21-24 Thus, Foxp3 is thought to be a suitable single marker for detecting CD4+CD25+ Tregs.
In murine models, Tregs inhibit the antitumor immune response mediated by CD4+CD25- T cells and CD8+ cytotoxic T cells.25-28 Population of Tregs in tumor infiltrating lymphocytes are significantly larger than in normal tissue in several malignancies.16-20 Moreover, Tregs infiltration is correlated with tumor progression in pancreatic ductal adenocarcinoma and gastric cancer.19,20 In this study, the population of Tregs were also increased in Bowen's disease and cutaneous squamous cell carcinoma compared to actinic keratosis. Thus, Tregs may highly be relevant in several human cancer progression. Since high Tregs infiltration reduces the anti-tumor immunity, Tregs infiltration has been suggested to be poor prognostic factor in several malignancies.17,20,29-31 On the contrary, recent studies showed that clinical outcome is not dependent on Tregs infiltration in renal cell cancer and colon cancer.32,33 Moreover, there is close correlation between Tregs infiltration and good survival in follicular lymphoma.34 The present study revealed that Tregs infiltration was not correlated with Ki67 LI in squamous cell carcinoma. Overall, it remains controversial as to whether Tregs infiltration is related to clinical behaviour.
The present study revealed that DCs infiltration was significantly in Bowen's disease and squamous cell carcinoma compared to actinic kertatosis. Moreover, the number of DCs was strongly related to Tregs infiltration, and DC stayed in close proximity to Tregs. A recent report showed that S100 positive DCs infiltration were correlated with Tregs infiltration in colon cancers, and also demonstrated that S100 positive DCs was primarily immature DCs.35 The function of DC effector strictly depend on both maturation stage and on environmental, anti-inflammatory or pro-inflammatory signals.36 Mature DCs have been regarded as inducers of T-cell immunity and vigorous T-cell proliferative responses, whereas immature DCs have been considered as inducers of T reg cells with inherent low ability to proliferate in response to mitogens or antigens.36 It is known that RANK and RANKL are key regulators of bone remodeling, mammary gland formation, lymph node development and T-cell/DC communication.6 RANKL overexpression in keratinocytes resulted in functional alterations of epidermal DCs and systemic increases of Tregs.6 RANK-RANKL signaling between RANK-expressing epidermal DCs and RANKL-overexpressing keratinocytes in K14-RANKL transgenic epidermis resulted in the up-regulation of DEC205 on epidermal DCs, a marker that has previously been associated with the induction of a tolerogenic phenotype in DCs.7 Moreover, DCs co-cultured with Tregs may also down-regulate the expression of costimulatory molecules, release large quantities of IL-10 and become unable to properly trigger T-cell activation.37,38 Recent studies also demonstrated direct contact of Treg with DC in vivo.39-41 Taken together, Tregs may act by directly targeting effector T cells, by competing with pathogenic T cells for access to antigen presenting cells (APCs) or by directly targeting APCs.
In conclusion, this study shows that Tregs are related to cutaneous squamous tumor progression.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download