Abstract
Purpose
Gastric carcinoma tissues release high level of prostaglandin E2 (PGE2) when compared to non-neoplastic mucosa, and cyclooxygenase-2 (COX-2), which is the rate-limiting enzyme in prostaglandin (PG) biosynthesis, is often overexpressed in gastric carcinomas and during gastric carcinogenesis. However, little is known about the expression of 15-hydroxyprostaglandin dehydrogenase (15-PGDH), the key enzyme responsible for the biological inactivation of PG, in gastric carcinomas.
Materials and Methods
We investigated the expression of 15-PGDH in 28 cases of advanced gastric carcinomas by Western blot analysis and also the relation between its expression and the gene promoter methylation.
Results
15-PGDH expression was significantly decreased in gastric carcinomas compared to corresponding non-neoplastic tissues and inversely correlated with the expression of proliferating cell nuclear antigen in gastric carcinomas. However, there was no correlation between 15-PGDH expression and pathological findings such as nodal metastasis and vascular invasion. Promoter hypermethylation of 15-PGDH gene was not detected in carcinomas, with only a negligible expression of the enzyme.
Prostaglandin E2 (PGE2) is a major prostanoid in gastric tissue and involved in various physiologic and pathologic functions of gastric mucosal cells.1 Gastric carcinoma tissues release high level of PGE2 compared to non-neoplastic mucosa.2 PGE2 is related to carcinogenesis through immunosuppression, inhibition of apoptosis, increase of metastatic potential of epithelial cells and promotion of angiogenesis.3-7 Overexpression of cyclooxygenase-2 (COX-2), the rate-limiting enzyme in prostaglandin (PG) biosynthesis, can be found in gastric carcinomas and during gastric carcinogenesis.8-11 PGs are rapidly metabolized by oxidation of 15(S)-hydroxyl group of PGs which is catalyzed by NAD+-linked 15-hydroxyprostaglandin dehydrogenase (15-PGDH).12 Recent studies identified a tumor suppressor activity of 15-PGDH in colon, lung, and breast carcinomas and suggested epigenetic silencing of the enzyme by DNA methylation and histone modification.13-17 However, little is known about the expression of 15-PGDH in gastric carcinoma. Moreover, the result of 15-PGDH expression in gastric carcinoma is controversial. One study reported that 15-PGDH expression was not altered in gastric carcinomas.18 On the other hand, 15-PGDH was found to be dysregulated in gastric carcinomas.14 Here, we investigated the expression of 15-PGDH in gastric carcinomas and their non-neoplastic tissues, and found that 15-PGDH expression was decreased in gastric carcinomas.
We used 28 frozen tissues of advanced gastric carcinomas (14 intestinal type and 14 diffuse type) and corresponding adjacent non-neoplastic tissues for Western blot analysis. Twenty-two paraffin embedded gastric carcinoma tissues were also used for immunohistochemical staining. The vascular invasion and nodal metastasis in gastric carcinomas were examined. The age distribution was between 45 and 65 years old, and male and female ratio was 1.5 : 1.
Formalin fixed paraffin embedded tissue sections of 4 µm thickness were made and spread on poly-L-lysine coated slides. The sections were deparaffinized and hydrated in a graded series of alcohol. Antigen retrieval was routinely performed by immersing the sections in 0.01 M citrate buffer (pH 6.0) in a pressure cooker by autoclaving for 15 minutes. Endogenous peroxidase activity was blocked with 3% hydrogen peroxide for 15 minutes and then incubated with primary antibody for 2 hours at room temperature. The primary antibody used was rabbit polyclonal anti-15-PGDH (Cayman Chemical, Ann Arbor, MI, USA, dilution 1:400). Staining was done with a DAKO LSAB+kit labeled with peroxidase (DakoCytomation, Carpinteria, CA, USA) and developed with 3, 3'-diaminobenzidine tetrahydrochloride (Zymed Laboratories, South San Franciso, CA, USA) as a chromogen. Sections were counterstained for 3 minutes with Mayer's hematoxylin and then mounted. As a negative control, rabbit IgG isotype was used instead of primary antibody.
Tissues were suspended in a lysis buffer [10 mM Tris-HCl (pH 7.4), 1 mM EDTA, and 0.25 M sucrose, 1% Triton X-100] supplemented with Complete mini protease inhibitor mixture tablets (Boehringer Mannheim, Mannheim, Germany) on ice for 1 hour. After removal of cell debris by centrifugation, protein concentration in cell lysates was determined by BCA protein assay reagent (Pierce, Rockford, IL, USA) with bovine serum albumin as a standard. Forty µg of protein were resolved by 12% SDS-polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane. The membranes were blocked with 5% skim milk in Tris buffered saline (TBS) for 1 hour at room temperature and probed overnight with antibodies at 4℃. The antibodies used were anti-15-PGDH (Cayman Chemical), anti-proliferating cell nuclear antigen (PCNA, Santa Cruz Biotechnology, Santa Cruz, CA, USA) and anti-β-actin (Santa Cruz Biotechnology). After washing with TBS-0.05% Tween 20, the blots were treated with horseradish peroxidase-conjugated anti-rabbit IgG antibody (1 : 3000; Zymed Laboratories) for 1 hour at room temperature. Western blots were developed by chemiluminescence (Pierce) and autoradiography was used for detection. Optical density was measured by using Scion Image (Scion Corp., Frederick, MD, USA).
Using a DNA Mini kit (Qiagen, Hilden, Germany), genomic DNA was isolated from 5 frozen tissues of gastric carcinomas and corresponding adjacent non-neoplastic tissues according to the manufacturer's instructions. DNA samples were modified by sodium bisulfite as described previously.19 Modified DNA was subjected to MSP. Briefly, genomic DNA is modified with sodium bisulfite, which converts all unmethylated cytosines to uracils, while 5-methylcytosines remain unaltered. Primers are designed to specifically amplify each of the sequences based on these chemically induced differences. The primers were as follows: unmethylated 15-PGDH (195 bp), sense 5-GGG TAT AAA AGT TGT GGT TGT GT-3, antisense 5-AAA AAA ATT TCC ACA ACT AAA CAC CA-3 and methylated 15-PGDH (189 bp), sense 5-GTA TAA AAG TCG CGG TCG CGC-3, antisense 5-AAA TTT CCG CGA CTA AAC GCC G-3.20 PCR conditions were as follows: 94℃ for 5 minutes, 30 amplification cycles (94℃ for 1 minute, 65℃ for 1 minute, 72℃ for 1 minute), followed by an additional extension step at 72℃ for 75 minutes. PCR products were electrophoresed in 1% agarose gel containing ethidium bromide and were photographed under ultraviolet light.
Adjacent non-neoplastic epithelial cells and carcinoma cells showed similarly strong cytoplasmic immunoreactivity for 15-PGDH (Fig. 1A). No difference was found between carcinomas and non-neoplastic tissues. Western blot analysis was done in order to verify the result of immunohistochemical staining. As shown in Fig. 1B, variable sized proteins, including a molecular weight of 25 kDa, were expressed in carcinoma and non-neoplastic tissue. We performed Western blot analysis to examine 15-PGDH expression in 28 gastric carcinomas and corresponding non-neoplastic tissues. The expression of 15-PGDH was significantly higher in non-neoplastic tissues than in carcinomas (Fig. 2, p < 0.05) and correlated inversely with the PCNA expression in gastric carcinomas (Fig. 3, p < 0.05, R = - 0.55). We examined the relationship between 15-PGDH expression level and vascular invasion and nodal metastasis in 22 cases of 28 gastric carcinomas to investigate the biologic behavior of the enzyme. Expression of 15-PGDH did not correlate with degree of the nodal metastasis and vascular invasion in gastric carcinomas (Fig. 4, p > 0.05). A recent study suggested epigenetic silencing of the enzyme by DNA methylation.17 Therefore, MSP was done in 5 gastric carcinomas with variable expression of the enzyme. As shown in Fig. 5, we could not detect the promoter methylation of 15-GDH in two gastric carcinomas with negligible expression of the enzyme (T1 and T3).
In this study, we observed that 15-PGDH expression was significantly decreased in gastric carcinomas compared to corresponding non-neoplastic tissues, and that the promoter hypermethylation of 15-PGDH gene was not detected in carcinomas with negligible expression of the enzyme.
A number of studies have reported up-regulation of COX-2, the rate-limiting enzyme in PG biosynthesis, in gastric carcinomas and during gastric carcinogenesis,8-11 but the expression of 15-PGDH, the key enzyme responsible for the biological inactivation of PG, in gastric carcinoma has not been studied in detail. Moreover, the results of 15-PGDH expression in 2 recent studies are contrary to each other.14,18 Therefore, we analyzed the expression of the enzyme by immunohischemical staining. 15-PGDH was well expressed in both gastric carcinoma cells and adjacent gastric mucosal epithelial cells, however, there was no difference between carcinomas and non-neoplastic tissues. On, Western blot analysis, variable sized proteins, including a molecular weight of 25 kDa, were expressed in carcinoma and non-neoplastic tissue. This may explain the result that immunohistochemistry did not show any difference in 15-PGDH expression between carcinomas and non-neoplastic tissues, and also the recent immunohistochemical study that 15-PGDH expression was not altered in gastric carcinomas.18 Therefore, immunohistochemical analysis was unable to reveal the difference of 15-PGDH expression level between carcinoma and non-neoplastic tissue. We performed Western blot analysis to examine 15-PGDH expression level, and found that the expression of 15-PGDH was significantly higher in non-neoplastic tissues than in carcinomas and inversely correlated with PCNA expression in gastric carcinomas. Similarly, 15-PGDH expression has been found to be highly expressed in normal colonic epithelia, but nearly undetectable in colon carcinomas.13,14 In addition, 15-PGDH transcript is decreased in gastric carcinomas compared with non-neoplastic gastric tissues.14 Recent studies demonstrated 15-PGDH to have tumor suppressor activity in colon, lung and breast carcinoma.13-17 These findings suggest that 15-PGDH has tumor suppressor activity also in gastric carcinoma.
Methylation of 15-PGDH promoter in breast and prostate carcinoma cells and tissues was shown.17,20 In prostate carcinoma cells, CpG methylation of PGDH was not accompanied by decreased gene expression, and expression was even induced in 3 prostate carcinoma samples, clearly showing CpG methylation.20 These results indicate that CpG methylation of a promoter should not be interpreted as a proof of its transcriptional repression. In this study, we did not detect hypermethylation of the gene promoter in carcinomas with negligible expression of the enzyme. The altered regulation of genes through up-regulation of zinc-finger transcriptional repressors, such as ZEB1, Slug, and Snail, is increasingly being recognized as an important mechanism in carcinoma progression.21 In human colon cancers, elevated Snail expression correlates well with down-regulation of 15-PGDH.22 In the future, relationship between the repressors and 15-PGDH expression should be analyzed in gastric carcinoma.
Figures and Tables
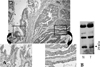 | Fig. 1Immunohistochemical staining and Western blot analysis of 15-PGDH in gastric carcinomas (T) and corresponding non-neoplastic tissue (N). (A) 15-PGDH was strongly expressed in both the cytoplasm of carcinoma cells and non-neoplastic mucosal epithelial cells. (B) Forty µg of protein was resolved by 10% SDS-polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane. 15-PGDH, 15-hydroxyprostaglandin dehydrogenase |
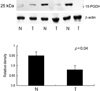 | Fig. 2Western blot analysis of 15-PGDH in gastric carcinomas (T) and corresponding non-neoplastic tissues (N). The expression of 15-PGDH was significantly higher in corresponding non-neoplastic tissues than in carcinomas (p < 0.05). Forty µg of protein was resolved by 10% SDS-polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane. The bottom represents β-actin, which was used as a loading control. 15-PGDH, 15-hydroxyprostaglandin dehydrogenase. |
 | Fig. 3Western blot analysis of 15-PGDH and PCNA in gastric carcinomas. 15-PGDH expression was inversely correlated with PCNA expression in gastric carcinomas (p < 0.05, R = - 0.55). Forty µg of protein was separated by 10% SDS-polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane. The bottom represents β-actin, which was used as a loading control. 15-PGDH, 15-hydroxyprostaglandin dehydrogenase; PCNA, proliferating cell nuclear antigen. |
 | Fig. 4Correlation between 15-PGDH expression, nodal metastasis and vascular invasion in gastric carcinomas. Expression of 15-PGDH was not related to the nodal metastasis and vascular invasion in gastric carcinomas (p > 0.05). 15-PGDH, 15-hydroxyprostaglandin dehydrogenase. |
 | Fig. 5Western blot analysis of 15-PGDH in gastric carcinomas and methylation specific PCR with methylated 15-PGDH primer sets (M) and unmethylated 15-PGDH primer sets (U) in gastric carcinomas. The promoter methylation of 15-PGDH was not observed in 2 gastric carcinomas (T1 and T3) with negligible expression of the enzyme. 15-PGDH, 15-hydroxyprostaglandin dehydrogenase. |
References
1. Eberhart CE, Dubois RN. Eicosanoids and gastrointestinal tract. Gastroenterology. 1995. 109:285–301.
2. Uefuji K, Ichikura T, Mochizuki H. Cyclooxygenase-2 expression is related to prostaglandin biosynthesis and angiogenesis in human gastric cancer. Clin Cancer Res. 2000. 6:135–138.
3. DeWitt DL. Prostaglandin endoperoxide synthase: regulation of enzyme expression. Biochim Biophys Acta. 1991. 1083:121–134.

4. Jones MK, Wang H, Peskar BM, Levin E, Itani RM, Sarfeh IJ, et al. Inhibition of angiogenesis by nonsteroidal anti-inflammatory drugs: insight into mechanisms and implications for cancer growth and ulcer healing. Nat Med. 1999. 5:1418–1423.

5. Tsujii M, DuBois RN. Alterations in cellular adhesion and apoptosis in epithelial cells overexpressing prostaglandin endoperoxide synthase 2. Cell. 1995. 83:493–501.

6. Tsujii M, Kawano S, DuBois RN. Cyclooxyenase-2 expression in human colon cancer cells increases metastatic potential. Proc Natl Acad Sci U S A. 1997. 94:3336–3340.

7. Tsujii M, Kawano S, Tsuji S, Sawaoka H, Hori M, DuBois RN. Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell. 1998. 93:705–716.

8. Ristimäki A, Honkanen N, Jänkälä H, Sipponen P, Härkönen M. Expression of cyclooxygenase-2 in human gastric carcinoma. Cancer Res. 1997. 57:1276–1280.
9. Murata H, Kawano S, Tsuji S, Tsuji M, Sawaoka H, Kimura Y, et al. Cyclooxygenase-2 overexpression enhances lymphatic invasion and metastasis in human gastric carcinoma. Am J Gastroenterol. 1999. 94:451–455.
10. Lim HY, Joo HJ, Choi JH, Yi JW, Yang MS, Cho DY, et al. Increased expression of cyclooxygenase-2 protein in human gastric carcinoma. Clin Cancer Res. 2000. 6:519–525.
11. Jang TJ. Expression of proteins related to prostaglandin E2 biosynthesis is increased in human gastric cancer and during gastric carcinogenesis. Virchows Arch. 2004. 445:564–571.

12. Tai HH, Ensor CM, Tong M, Zhou H, Yan F. Prostaglandin catabolizing enzymes. Prostaglandins Other Lipid Mediat. 2002. 68-69:483–493.

13. Yan M, Rerko RM, Platzer P, Dawson D, Willis J, Tong M, et al. 15-Hydroxyprostaglandin dehydrogenase, a COX-2 oncogene antagonist, is a TGF-bata-induced suppressor of human gastrointestinal cancers. Proc Natl Acad Sci U S A. 2004. 101:17468–17473.

14. Backlund MG, Mann JR, Holla VR, Buchanan FG, Tai HH, Musiek ES, et al. 15-Hydroxyprostaglandin dehydrogenase is down-regulated in colorectal cancer. J Biol Chem. 2005. 280:3217–3223.

15. Ding Y, Tong M, Liu S, Moscow JA, Tai HH. NAD+-linked 15-hydroxyprostaglandin dehydrogenase (15-PGDH) behaves as a tumor suppressor in lung cancer. Carcinogenesis. 2005. 26:65–72.

16. Myung SJ, Rerko RM, Yan M, Platzer P, Guda K, Dotson A, et al. 15-Hydroxyprostaglandin dehydrogenase is an in vivo suppressor of colon tumorigenesis. Proc Natl Acad Sci U S A. 2006. 103:12098–12102.
17. Wolf I, O'Kelly J, Rubinek T, Tong M, Nguyen A, Lin BT, et al. 15-hydroxyprostaglandin dehydrogenase is a tumor suppressor of human breast cancer. Cancer Res. 2006. 66:7818–7823.

18. Yoo NJ, Jeong EG, Lee SH, Lee SH. Expression of 15-hydroxyprostaglandin dehydrogenase, a COX-2 antagonist and tumour suppressor, is not altered in gastric carcinomas. Pathology. 2007. 39:174–175.

19. Herman JG, Graff JR, Myohanen S, Nelkin BD, Baylin SB. Methylation-specific PCR: a novel PCR assay for methylation status of CpG island. Proc Natl Acad Sci U S A. 1996. 93:9821–9826.

20. Lodygin D, Epanchintsev A, Menssen A, Diebold J, Hermeking H. Functional epigenomics identifies genes frequently silenced in prostate cancer. Cancer Res. 2005. 65:4218–4227.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download