Abstract
Extranodal marginal zone lymphoma is a low-grade B cell lymphoma that presents with an indolent clinicopathologic nature. Although this tumor can occur in various sites, including the gastrointestinal tract and lungs, it develops and spreads extremely rarely along the trachea and central airway. We report a case of extranodal lymphoma of mucosa-associated lymphoid tissue with tracheobronchial involvement. An 83-year-old woman presented with a cough and dyspnea. Bronchoscopic evaluation confirmed diffuse, multiple nodular lesions in both the trachea and large bronchi, and she was diagnosed with an extranodal marginal zone lymphoma of the tracheobronchial tree. After systemic chemotherapy, she survived for more than 18 months.
Extranodal marginal zone lymphomas (EMZLs) were first described by Isaacson and Wright in 1983.1 They may affect any organ in the body, including the gastrointestinal tract, lungs, salivary glands, orbit, thyroid, skin, breast, and urinary bladder.2,3 However, EMZLs of mucosa-associated lymphoid tissue occurring in the trachea is extremely rare, regardless of whether it is primary involvement or secondary dissemination.3,4 With lung involvement, most pulmonary EMZLs demonstrate alveolar or nodular patterns on chest computed tomography (CT).5-9 Therefore, it is uncommon for EMZLs of mucosa-associated lymphoid tissue to initially present as tracheobronchial involvement.10 Here, we describe a patient with an extranodal lymphoma of mucosa-associated lymphoid tissue, mainly occurring along the trachea and large bronchi, which caused luminal narrowing of the central airway.
An 83-year-old woman presented with a dry cough and dyspnea for 2 months duration. She had an 8-kg weight loss over that period, but had not experienced night sweats or fever. Her medical, smoking, and family histories were all noncontributory.
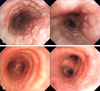
On admission, her physical examination, including ear, nose, and throat was unremarkable. No abnormal laboratory findings were detected. Chest CT showed diffuse narrowing of the tracheobronchial tree with endobronchial nodularities, resulting in the collapse of the right middle lobe (RML) and small peripheral nodules in both lungs (Fig. 1). At bronchoscopy, multiple, variable-sized nodules causing bronchial narrowing were seen from the proximal trachea to bronchi of both lungs, including the carina (Fig. 2). Specimens obtained from the right-second carina revealed small monophasic lymphoid tumor cells, diffusely infiltrating the bronchial mucosa, and characteristic lymphoepithelial lesions (Fig. 3). Immunohistochemical analysis of these lesions indicated uniform presence of B-cell markers CD20 and CD 79a, the absence of CD3, CD5, CD23, and CD45RO, and positive staining for anti-kappa light chain. Therefore, the lesion was compatible with an EMZL of mucosa-associated lymphoid tissue.
We performed CT of the abdomen and neck, gastrofiberscopy, and a bone marrow biopsy to establish the stage. The bone marrow biopsy and gastrofiberscopy indicated no lymphoma involvement. In contrast, CT of the neck and abdomen showed multiple, variable-sized lymphadenopathies in the cervical area and left pelvic cavity. Ann Arbor stage IIIE was diagnosed, and 6 cycles of CVP (cyclophosphamide, vincristine, and prednisolone) chemotherapy were administered. After the treatment, follow-up CT of the chest and abdomen showed improvement of the diffuse bronchial narrowing and multiple lymphadenopathies. At that time, bronchoscopy was performed to evaluate pneumonia. However, we found no abnormal mucosal lesion in the tracheobronchial tree or evidence of lymphoma recurrence in a bronchial biopsy.
EMZLs originate from B cell lymphocytes of the marginal zone in mucosa-associated lymphoid tissue (MALT). The presence of MALT in the lung was first described by Bienenstock et al. in 1973, however, MALT is not a constitutive structure in the respiratory tract of healthy adults.11,12 Some studies have shown that MALT develops in the areas of antigen exposure and protects the mucosa by taking up antigens and participating in the immune mechanism.13
Primary lymphoma, especially MALT lymphoma, arising in the trachea is very uncommon.3,4,14-19 To the best of our knowledge, only 5 cases of confirmed EMZL of the trachea have been reported.3,14-17 Based on features of extranodal lymphoma of MALT, most cases show 1 or a few nodular lesions localized within the trachea, but not systemic dissemination. In our case, however, MALT lymphoma occurred simultaneously in the trachea and large airways, and systemic involvement was observed at the initial presentation. This tracheobronchial involvement, either primary or secondary, is also rare among patients with lymphoma.
The lung involvement of an EMZL usually spreads along the bronchovascular bundles and interlobular septa.8 Although no specific radiographic findings have been established for MALT lymphoma of the lung, many studies have reported that pulmonary nodules and air space consolidation are major CT patterns.5-9 In the most recent published report on CT findings of MALT lymphoma of the lungs,9 single or multiple nodules or consolidations comprised the main pattern, occurring in 76% of patients, while none exhibited involvement of the upper respiratory tract. Therefore, our case is unusual because the patient initially presented with a characteristic diffuse endobronchial nodular lesion, as seen on bronchoscopy and chest CT. This peculiar spreading pattern readily provided sufficient samples from the bronchoscopic mucosal biopsy. The lesion was consistent with a MALT lymphoma in that it had monophasic small lymphocytes diffusely infiltrating the bronchial mucosa, which is a characteristic lymphoepithelial lesion, and an immunophenotype showing only B-cell markers and monotypic anti-kappa light chain positive.
No guidelines for the optimal management of tracheobronchial MALT lymphomas have been established.2,3 Several treatment options exist, such as surgical resection, radiation, and chemotherapy, including anti-CD 20 monoclonal antibody, alone or in combination, as well as therapeutic abstention. Since our patient had extrapulmonary involvement and was elderly, she was treated with the standard CHOP chemotherapy regimen without adriamycin.
In summary, our patient presented with a cough and dyspnea and a thorough bronchoscopic evaluation confirmed an EMZL occurring in both the trachea and large bronchi. After systemic chemotherapy, she showed a complete response and survived for more than 18 months. We should consider the possibility of EMZL in differentiating tracheobronchial nodular lesions, because of specific features of the disease, including an excellent prognosis.
Figures and Tables
Fig. 1
CT of the chest shows diffuse narrowing of the tracheobronchial tree with endobronchial nodularities and resultant collapse of the RML (A), and presence of peripheral nodules in both lungs (B). CT, computed tomography, RML, right middle lobe.

References
1. Isaacson P, Wright DH. Malignant lymphoma of mucosa-associated lymphoid tissue. A distinctive type of B-cell lymphoma. Cancer. 1983. 52:1410–1416.

2. Thieblemont C, Berger F, Dumontet C, Moullet I, Bouafia F, Felman P, et al. Mucosa-associated lymphoid tissue lymphoma is a disseminated disease in one third of 158 patients analyzed. Blood. 2000. 95:802–806.

3. Zinzani PL, Magagnoli M, Galieni P, Martelli M, Poletti V, Zaja F, et al. Nongastrointestinal low-grade mucosa-associated lymphoid tissue lymphoma: analysis of 75 patients. J Clin Oncol. 1999. 17:1254.
4. Fidias P, Wright C, Harris NL, Urba W, Grossbard ML. Primary tracheal non-Hodgkin's lymphoma. A case report and review of the literature. Cancer. 1996. 77:2332–2338.

5. Ahmed S, Kussick SJ, Siddiqui AK, Bhuiya TA, Khan A, Sarewitz S, et al. Bronchial-associated lymphoid tissue lymphoma: a clinical study of a rare disease. Eur J Cancer. 2004. 40:1320–1326.

6. Cordier JF, Chailleux E, Lauque D, Reynaud-Gaubert M, Dietemann-Molard A, Dalphin JC, et al. Primary pulmonary lymphomas. A clinical study of 70 cases in nonimmunocompromised patients. Chest. 1993. 103:201–208.
7. Lee DK, Im JG, Lee KS, Lee JS, Seo JB, Goo JM, et al. B-cell lymphoma of bronchus-associated lymphoid tissue (BALT): CT features in 10 patients. J Comput Assist Tomogr. 2000. 24:30–34.

8. Sankaranarayanan V, Zeidalski TM, Chitkara RK. A 55-year-old smoker with a persistent right lower lobe infiltrate. Chest. 2005. 127:2266–2270.

9. Bae YA, Lee KS, Han J, Ko YH, Kim BT, Chung MJ, et al. Marginal zone B-cell lymphoma of bronchus-associated lymphoid tissue: imaging findings in 21 patients. Chest. 2008. 133:433–440.

10. Solomonov A, Zuckerman T, Goralnik L, Ben-Arieh Y, Rowe JM, Yigla M. Non-Hodgkin's lymphoma presenting as an endobronchial tumor: report of eight cases and literature review. Am J Hematol. 2008. 83:416–419.

11. Bienenstock J, Johnston N, Perey DY. Bronchial lymphoid tissue. I. Morphologic characteristics. Lab Invest. 1973. 28:686–692.
12. Pabst R. Is BALT a major component of the human lung immune system? Immunol Today. 1992. 13:119–122.

13. Sminia T, van der Brugge-Gamelkoorn GJ, Jeurissen SH. Structure and function of bronchus-associated lymphoid tissue (BALT). Crit Rev Immunol. 1989. 9:119–150.
14. Okubo K, Miyamoto N, Komaki C. Primary mucosa-associated lymphoid tissue (MALT) lymphoma of the trachea: a case of surgical resection and long term survival. Thorax. 2005. 60:82–83.

15. Kaplan MA, Pettit CL, Zukerberg LR, Harris NL. Primary lymphoma of the trachea with morphologic and immunophenotypic characteristics of low-grade B-cell lymphoma of mucosa-associated lymphoid tissue. Am J Surg Pathol. 1992. 16:71–75.

16. Wiggins J, Sheffield E, Green M. Primary B cell malignant lymphoma of the trachea. Thorax. 1988. 43:497–498.

17. Tsurutani J, Kinoshita A, Kaida H, Fujii H, Narasaki F, Fukuda M, et al. Bronchoscopic therapy for mucosa-associated lymphoid tissue lymphoma of the trachea. Intern Med. 1999. 38:276–278.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download