Abstract
Epidermal growth factor has an important role in the regulation of proliferation and differentiation in epidermal keratinocytes, as well as in the survival, angiogenesis and metastasis of cancer cells. Cetuximab is a chimeric monoclonal antibody selective for the epidermal growth factor receptor that induces a broad range of cellular responses that enhance tumor sensitivity to radiotherapy and chemotherapeutic agents. However, it can cause adverse events in the patient including acneiform eruption, asthenia, abdominal pain and nausea/vomiting. We report a case of severe acneiform eruption induced by cetuximab in a 56-year-old man with colorectal cancer and liver metastases.
Cetuximab is a chimeric monoclonal antibody highly selective for the epidermal growth factor receptor (EGFR), which has demonstrated efficacy in colorectal, head and neck and non-small-cell lung cancer. Common adverse events associated with cetuximab therapy are acneiform eruption, asthenia, abdominal pain and nausea/vomiting.1 Acneiform eruption is the most common adverse effect with > 60% of incidence and 5.2 - 18% of grade 3/4 adverse events reported in the clinical trials.1-3 Here we report an extensive and severe acneiform eruption induced by cetuximab.
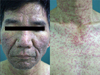
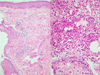
A 56-year-old man was admitted for skin lesions on the face and upper trunk, which had developed five days previous. He had previously undergone therapy with cetuximab (Erbitux®) 250 mg/m2 weekly (at the first infusion, loading dose was 400 mg/m2) and irinotecan (Campto®) 100 mg/m2 weekly for six consecutive weeks (followed by two weeks rest) for colorectal cancer with liver metastases. He did not complain of any itching or pain. Physical examination revealed multiple rice-sized erythematous pustules on the face and upper trunk (Fig. 1). Histopathologic finding was folliculitis in which periadnexal neutrophilic infiltrate was prominent (Fig. 2). Acneiform eruptions were resolved after treatment with oral ciprofloxacin and topical mupirocin ointment (Bactroban®) for two weeks.
EGFR is a transmembrane receptor possessing tyrosine kinase activity which is stimulated by growth factors such as epidermal growth factor (EGF). EGF is crucial for tumor cell proliferation, inhibition of apoptosis, and other processes important for cancer progression; including angiogenesis, invasion and metastasis.1,2,4 Inhibitors of EGFR are monoclonal antibodies against EGFR (e.g. cetuximab, panitumumab) or EGFR tyrosine kinase inhibitors (e.g. gefitinib, erlotinib).2
Cetuximab has a higher affinity for EGFR than EGF and competitively blocks the cellular action of these naturally occurring ligands.1 Cetuximab has also been shown to promote receptor internalization, thereby down-regulating EGFR.1,4
Cetuximab is used in many epithelial cancers that overexpress EGFR, including head and neck, breast, colon, lung, prostate, kidney, ovarian, brain, pancreatic, and bladder cancers.5 Among these, cetuximab is most commonly prescribed for metastatic colorectal cancer, administered alone or in combination with irinotecan-based chemotherapy regimens.1
Skin reactions following cetuximab treatment are acneiform eruption, xerosis/desquamation, paronychia, hair changes, telangiectasia and hyperpigmentation.2,6,7 Acneiform skin eruption is the most common adverse event associated with cetuximab therapy.1-3,6,7 The resultant rash is predominantly on the seborrheic areas such as face, neck, retroauricular area, shoulders, and upper trunk.2,6 The skin lesions sometimes consist of itchy erythematous follicular papules that may evolve into pustules. The follicular skin lesions are not preceded by visible comedones and can therefore not be considered true acne.2 This adverse effect is thought to be due to its interference with the physiological role of EGF in the epidermis.1,2,5,7 EGFR is important for autocrine regulation of the growth of the epidermis, cell cycle progression, cell differentiation, cell movement, and cellular survival.5
Histopathology is characterized by a superficial and florid neutrophilic suppurative folliculitis in which a dense monomorphic infiltrate of neutrophils is predominant around the infundibula.3
Investigation of the follicular toxic effects of cetuximab is important not only because cetuximab is a novel antitumor agent that is likely to gain widespread use, but also because of its in vivo confirmation of the significant role of EGFR in follicular homeostasis.
Although acneiform eruption caused by cetuximab is a relatively common adverse reaction, it may be worthwhile to pay close attention to this patient who presented a severe and extensive eruption.
Figures and Tables
References
1. Reynolds NA, Wagstaff AJ. Cetuximab: in the treatment of metastatic colorectal cancer. Drugs. 2004. 64:109–118. discussion 119-21.
2. Segaert S, Van Cutsem E. Clinical signs, pathophysiology and management of skin toxicity during therapy with epidermal growth factor receptor inhibitors. Ann Oncol. 2005. 16:1425–1433.

3. Jacot W, Bessis D, Jorda E, Ychou M, Fabbro M, Pujol JL, et al. Acneiform eruption induced by epidermal growth factor receptor inhibitors in patients with solid tumours. Br J Dermatol. 2004. 151:238–241.

4. Kimyai-Asadi A, Jih MH. Follicular toxic effects of chimeric anti-epidermal growth factor receptor antibody cetuximab used to treat human solid tumors. Arch Dermatol. 2002. 138:129–131.

5. Monti M, Mancini LL, Ferrari B, Rahal D, Santoro A. Complications of therapy and a diagnostic dilemma case. Case 2. Cutaneous toxicity induced by cetuximab. J Clin Oncol. 2003. 21:4651–4653.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download