Abstract
Purpose
Exposure of male reproductive organs to 2,3,7,8-Tetrachlorodibenzo-p-Dioxin (TCDD) has been reported to cause developmental changes. In this study, we evaluated the effects of in utero TCDD exposure on male reproductive development.
Materials and Methods
Pregnant C57BL/6 mice were administered a single intraperitoneal injection of TCDD (1 µg/kg) on gestation day (GD) 15. The offspring were examined in the immature stage on postnatal day (PND) 30 and in the mature stage on PND 60. The testes were examined for histological changes, androgen receptor (AR), proliferating cell nuclear antigen (PCNA) and apoptosis following the measurement of morphological changes.
Results
Anogenital distance (AGD) and testis weights were reduced by TCDD exposure both on PND 30 and PND 60 while body weights and length of male offspring were not affected by TCDD. The regular sperm developmental stage was impaired with TCDD treatment on PND 30. However, no difference was found between the control group and TCDD groups on PND 60. Simultaneously, the expression of AR was also reduced on PND 30, while it was increased on PND 60 compared with the control group. The expression of PCNA was decreased whereas apoptosis was not affected by TCDD both on PND 30 and PND 60.
Incidences of urogenital anomali such as hypospadias, cryptorchidism, and testicular cancer have increased, and the environmental endocrine disrupters (EDs) may be responsible for these abnormalities.1 Most EDs are produced in industrial factories and released into the environment. Chemicals used in daily life, such as herbicides, are also major pollutants, and they are believed to be primarily absorbed through dietary intake and accumulate in animals and humans.2 One of the endocrine disrupters, 2,3,7,8-Tetrachlorodibenzo-p-Dioxin (TCDD), is the most toxic congener of a class of polyhalogenated aromatic hydrocarbons.3 TCDD has shown to induce toxic responses such as carcinogenicity in humans, and hepatotoxicity and teratogenicity in laboratory animals.4-6 Maternal exposure to TCDD was reported to cause reproductive changes in male offspring; reduced sperm count, decreased anogenital distance, reduced size of reproductive organs, and a feminized regulation of pituitary luteinizing hormone secretion.7-10 While there is a substantial amount of evidence to indicate that TCDD adversely affects reproductive male organs, the mechanism of this impairment is not well understood.
Androgens are necessary for male sexual development and maturation, and they serve to maintain reproductive organs.11 Down-regulation of androgen receptor (AR) mRNA or low immunoreactivity of AR caused by in utero and lactational TCDD exposure has been observed in the prostate.12-15 One possible explanation of alteration in male reproductive organs is that in utero and lactational TCDD exposure reduces androgen concentration and androgen receptor expression. The objective of this study is to examine the androgen receptor expression in testes after in utero TCDD exposure.
Germ cell maturation towards the center of the seminiferous tubules is required for spermatogenesis, which includes spermatogonia mitotic proliferation, spermatocytes meiotic division, spermatid differentiation, and spermatozoa release into the center of the tubule.16 Germ cell apoptosis also occurs normally during spermatogenesis in mammalian species.17 It has been reported that exposure to toxic agents such as phthalate, ethane dimethane sulfonate, zearalenone, and TCDD induces apoptosis in the seminiferous tubules.18-21 However, there are few studies to report the effects of EDs on the proliferation of testicular cells. Proliferating cell nuclear antigen (PCNA) is a component of DNA polymerase of proliferating somatic cells,22-24 and is expressed in proliferating spermatogonia and early spermatocytes.25 Thus, we focused our efforts on the effects of in utero TCDD exposure on spermatogenesis and investigated the mechanism of morphological alteration.
C57BL/6 mice (20 females and 10 males) were obtained from Daehan Biolink Co., LTD, Incheon, Korea, and were cared in the AAALAC system. The animal procedure was approved by the Institutional Animal Care and Use Committee of the Yonsei University College of Medicine. Two females were housed overnight with 1 male per cage, and the presence of a vaginal plug was checked on the following morning. The day when sperm plugs were found was considered gestation day 0 (GD 0). On GD 15, pregnant females (n = 5 for each group) were randomly chosen and given a single I.P. injection of TCDD (1 µg TCDD/kg body weight). The rest were given an equivalent volume of vehicle (corn oil, Sigma Chemical Co., St. Louis, MO, USA) as a control. This period was selected because it has previously been reported that a critical window for the impairment of male reproductive organs occurred around GD 15 when Long-Evans hooded rats or Sprague-Dawley rats were exposed to TCDD (1 µg TCDD/kg body weight) compared to GD 8 or GD 18 TCDD exposure.14,26 The male offspring were weighed and sacrificed on PND 30 and 60. Body length (the length between the tip of the nose and anterior edge of the anus) and anogenital distance (AGD, the distance from the anterior edge of the anus to the base of the genital tubercle) were measured with a caliper. Testes were excised, weighed, fixed in 10% formalin, and embedded in paraffin for further study.
Paraffin-embedded testes were sectioned into 4-µm-thickness. After deparaffinized and rehydrated with graded alcohols, the tissue sections were stained with hematoxylin and eosin (H-E) and examined for histological lesions.
To detect proliferating cell nuclear antigen (PCNA) in testes, the tissue sections were deparaffinized and rehydrated through graded alcohols. Thereafter, the sections were subjected to microwave antigen retrieval treatment for 20 minutes, and nonspecific binding was blocked by incubation with 10% normal swine serum (DAKO Corporation, Carpinteria, CA, USA) in TPBS (phosphate buffered saline with 10% triton X-100) for 30 minutes. Mouse monoclonal proliferating cell nuclear antigen antibody (PCNA, DAKO Corporation) was diluted in blocking solution and incubated over the sections in a humid chamber for 16 hours at 4℃. A biotinylated anti-mouse IgG was then applied, followed by streptavidin peroxidase (DAKO LSAB+ Kit, DAKO Corporation) treatment for 1 hour at room temperature (RT). Immunoreactions were localized using hydrogen peroxide-activated 3,3'-diaminobenzidine-tetrahydrochloride (Sigma Chemical Co, St. Louis, MO, USA). In each group, 1 testis from 5 offspring each from a different litter, was selected for evaluation. Three sections from different parts of each testis were chosen. A total of 10 seminiferous tubules in each section were randomly chosen, and the average number of PCNA-positive cells per tubule was analyzed.
For androgen receptor immunostaining, a tyramide amplification step was applied according to the manufacture's instructions (TSA Biotin system kit, PerkinElmer, Boston, MA, USA). Briefly, after tissue sections were deparaffinized and rehydrated through graded alcohols, antigen retrieval was performed by microwave for 10 minutes in 0.1 M citric acid buffer, pH 6.0. After cooling at RT, the endogenous peroxidase activity was blocked with 3% H2O2 in 50% methanol for 20 minutes, and avidin and biotin were blocked by the supplement provided in the kit. Next, tissues were incubated with the polyclonal anti-AR antibody (PG-21, Upstate, Lake Placid, NY, USA) for 16 hours at 4℃, followed by a 1-hour of incubation with biotinylated goat anti-rabbit antibody (Zymed Laboratories Inc, San Francisco, CA, USA). For biotin amplification, the tissue sections were incubated with biotinyl tyramide at RT for 10 minutes, followed by incubation with streptavidin-HRP at RT for 30 minutes. The immunoreactions were localized using hydrogen peroxide activated 3,3'-diaminobenzidine-tetrahydrochloride (Sigma Chemical Co). Positive reactions were examined under light microscope equipped with a CCD system. In each group, 1 testis from 5 offspring each from a different litter, was selected for evaluation.
For TUNEL assay, tissue sections were deparaffinized and rehydrated through graded alcohols, and then treated with proteinase K (15.1 µg/mL in 10 mM Tris-HCl, Roche, Mannheim, Germany) for 30 minutes at RT. The tissue sections were incubated with reaction buffer (In Situ Cell Death Detection Kit, POD, Roche, Mannheim, Germany) to label DNA strand breaks for 1 hour at 37℃ in the dark, and treated with horse-radish-peroxidase for 30 minutes at 37℃. The reaction was performed using DAB (Sigma Chemical Co) at RT, and the stained cells were analyzed under a light microscope. Apoptotic cells were identified by their deep blue color and condensed size. In each group, 1 testis from 5 offspring each from a different litter, was selected for evaluation.
The body weights and lengths of male offspring were not significantly affected by TCDD exposure when measured on PND 30 and PND 60. However, the absolute AGD and relative AGD levels were significantly decreased on PND 30. On PND 60, the absolute AGD was also decreased after TCDD exposure. TCDD also reduced the absolute weight of the testes and epididymis, both on PND 30 and on PND 60. The relative weights of each structure were significantly decreased on PND 60 compared with those of the control group. When the 2 TCDD exposed groups were compared, the percentage decrease of PND 60 was much lower than that of PND 30 (Table 1).
Control and TCDD-exposed testes on PND 30 and PND 60 (Fig. 1) were observed by hematoxylin and eosin-stained cross sections. On PND 30, the germ cell population in seminiferous tubules was destroyed in TCDD-exposed testes. None of the testes investigated on PND 60 exhibited any alteration.
Androgen receptor (AR) expression in testes on PND 30 and PND 60 was immunohistochemically examined. Positive reactivity was noted in nuclear staining. AR was present in most Leydig cells, Sertoli cells, and peritubular myoid cells in the testes. AR expression was decreased on PND 30 in TCDD-treated testes, but AR expression on PND 60 was increased in TCDD-exposed testes (Fig. 2).
PCNA positive cells were observed in spermatocytes and spermatogonia in seminiferous tubules of both control and TCDD-treated groups. The number of PCNA positive cells per seminiferous tubule was counted, and 10 circular formed seminiferous tubules per section from 5 different dams were examined. TCDD exposure reduced the number of PCNA positive cells on PND 30 and PND 60. The number of PCNA positive cells in GD 15 TCDD-treated testes was significantly decreased compared with that in the control group on both PND 30 and PND 60. However, when the 2 TCDD exposed groups were compared, the percentage of PND 60 decrease was much lower than that of PND 30 (Fig. 3).
TUNEL positive cells were identified by their condensed size, and the spermatocytes and spermatogonia were labeled in both control and TCDD exposed testes. Sertoli cells, Leydig cells, and other interstitial cells were TUNEL negative in both control and TCDD exposed testes. TCDD did not affect germ cell apoptosis on PND 30 and PND 60 (Fig. 4).
In this study, we examined the effects of in utero TCDD exposure on the development of the male offspring reproductive system. TCDD was found to adversely affect the development of the male offspring reproductive system by suppressing androgen function. In addition, we observed that the adverse effects of TCDD were attenuated with increasing time. This is the first report to demonstrate the self-repairing capability of the testes after TCDD treatment.
Morphological alterations are the most common adverse effects of TCDD exposure on the development of animals. Many studies have demonstrated the effects of TCDD on the morphological alteration of offspring in rodents, reporting that in utero or lactational TCDD exposure reduces the weight of male reproductive organs compared with that in the control group, while body weights are unaffected.9,13 Anogenital distance (AGD) has also been reported to decrease in utero or in lactational TCDD exposure.13,14,26 In our study, we also observed that the weight of the testes decreased after TCDD exposure, while body weight was not affected. Moreover, AGD was found to have decreased on PND 30 and PND 60 compared to that in the control group. However, the decrease on PND 60 was less than that on PND 30 (Table 1), suggesting that the effects of TCDD on testes tend to decrease after PND 60. Here we report for the first time that it is possible to correct the adverse effects of TCDD.
Upon histological examination, we found that TCDD destroyed many germ cells at every stage,evidenced by the lesions of testes on PND 30. However, on PND 60, no significant differences were found in the control or TCDD-treated groups (Fig. 1). In fact, the effect of TCDD on each stage of spermatogenesis in the testes still remains a matter of debate. El-Sabeawy et al. reported that the diameter of the seminiferous tubule was decreased and the spermatogonial population was significantly absent after TCDD exposure (10 µg/kg),27 and Faqi et al. reported that pathological changes were detected in individual seminiferous tubules, which included pyknotic nuclei and the occurrence of cell debris in the lumen.7 It should, however, be pointed out that most tubules were normal without any signs of direct damages. Other investigators indicated that, despite the TCDD-induced morphological alteration, there were no histological differences between the control and TCDD-treated groups.13,28 However, based on our data, we suggest that the lesion on testes may be attributed to reduced AR expression.
Androgens are hormones essential for developing and maintaining the cells in the male reproductive organs.11 The functions of androgens are completed by binding to the androgen receptor.29 It has been reported that suppression of AR during the gestational period results in a significant downregulation of testosterone, AGD and reproductive tissue weight as well as number of Sertoli cells.30-32 In the prostate, in utero and lactational exposure to TCDD reduces the expression of AR.12,15 on the other hand, it has been reported that TCDD treatment does not seem to have much influence on AR expression in the seminal vesicle.33 Judging from the reports, the effect of TCDD appears to vary depending on the type of tissue. We also found in this study that, the AR expression was increased on PND 60 compared to the control group although androgen receptor expression decreased on PND 30 (Fig. 2). In humans, the half life of TCDD has been reported to be from 7 to 10 years.34 We, therefore, speculate that the concentration of TCDD in the adult mouse (PND60) has already decreased to much less than that of the neonatal or juvenile period (PND 30). This may explain the fact that the AR rebounded on PND 60. Thus, the greater reduction of the level of AGD on PND 30 than on PND 60, and the recovery of testes on PND 60 seem to be related to the increased AR level on PND 60.
Another important finding of the present study was that in utero TCDD exposure suppressed the development of the reproductive system in the male offspring by suppressing cell proliferation rather than by inducing cell apoptosis. Although PCNA was significantly reduced both on PND 30 and PND 60 in TCDD-exposed groups, decrease of PCNA level was strikingly greater on PND 30 than on PND 60 (Fig. 3). PCNA expression has previously been shown to be dose-dependently related to androgens35 and androgens are regulated by AR. Therefore, the change of PCNA contributes to the alteration of AR. Nevertheless, we did not find induction of apoptosis in the two TCDD treated groups (Fig. 4). This is in contrast to the previous study by Schultz,21 and may be due to the difference in concentration, duration of treatment, and application methods.
In summary, the present study demonstrates that in utero exposure to TCDD can impair the development of offspring testes by suppressing the androgen receptor. However, the adverse effects of TCDD on male offspring showed a tendency to decrease over time. This trend of recovery can probably be due to coordinated action of androgen receptors and hither-to unknown mechanisms of TCDD-induced toxicity. These unknown mechanisms need to be investigated in future studies.
Figures and Tables
Fig. 1
Histological assessment of testis from male offspring In utero exposure to TCDD. (A and C) Testes from control group on PND 30 and PND 60, respectively. (B and D) Testes from TCDD exposed group on PND 30 and PND 60. n = 9 - 10 for each group (Magnification: × 100). PND, postnatal day; TCDD, 2,3,7,8-Tetrachlorodibenzo-p-Dioxin.
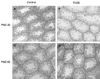
Fig. 2
Effects of in utero TCDD exposure on expression of androgen receptor. Pregnant mice were injected with 1 µg/kg TCDD concentration on GD 15. Testes on PND 30 and PND 60 were used to examine the expression of androgen receptor (AR) by immunohistochemistry (n = 5, for each group). AR was present in Leydig cells (white arrow), Sertoli cells (black arrow) and peritubular myoid cells (arrow head) in testis. (A and C) Testes from control group on PND 30 and PND 60 respectively. (B and D) Testes from TCDD exposed group on PND 30 and PND 60 (Magnification: × 100). PND, postnatal day; TCDD, 2,3,7,8-Tetrachlorodibenzo-p-Dioxin.
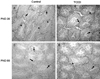
Fig. 3
Effects of in utero TCDD exposure on cell proliferation. Pregnant mice were injected with 1 µg/kg TCDD concentration on GD 15. Testes on PND 30 and PND 60 were used to examine cell proliferation using specific anti-PCNA by immunohistochemistry. Three sections from different parts of each testis were chosen, and a total of 10 seminiferous tubules in each section were randomly chosen for counting PCNA-positive cells. (A and C) Testes from control group on PND 30 and PND 60, respectively. (B and D) Testes from TCDD exposed group on PND 30 and PND 60. (E) The quantification of PCNA positive germ cells. Data indicate means ± SEM. n = 5 for each group. PND, postnatal day; TCDD, 2,3,7,8-Tetrachlorodibenzo-p-Dioxin. *p < 0.01, **p < 0.01 (Magnification: × 100).
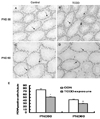
Fig. 4
Effects of in utero TCDD exposure on cell apoptosis. Pregnant mice were injected with 1 µg/kg TCDD concentration on GD 15. Testes from PND 30 and PND 60 were used to examine cell apoptosis TUNEL staining (arrow head) (n = 5 for each group). (A and C) Testis from control group on PND 30 and PND 60, respectively. (B and D) Testis from TCDD exposed group on PND 30 and PND 60 (Magnification × 200). PND, postnatal day; TCDD, 2,3,7,8-Tetrachlorodibenzo-p-Dioxin.
References
1. Chia SE. Endocrine disruptors and male reproductive function--a short review. Int J Androl. 2000. 3:45–46.
2. Schecter A, Cramer P, Boggess K, Stanley J, Päpke O, Olson J, et al. Intake of dioxins and related compounds from food in the U.S. population. J Toxicol Environ Health A. 2001. 63:1–18.

3. Safe SH. Comparative toxicology and mechanism of action of polychlorinated dibenzo-p-dioxins and dibenzofurans. Annu Rev Pharmacol Toxicol. 1986. 26:371–399.

4. Fingerhut MA, Halperin WE, Marlow DA, Piacitelli LA, Honchar PA, Sweeney MH, et al. Cancer mortality in workers exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. N Engl J Med. 1991. 324:212–218.

5. Goodman DG, Sauer RM. Hepatotoxicity and carcinogenicity in female Sprague-Dawley rats treated with 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD): a pathology working group reevaluation. Regul Toxicol Pharmacol. 1992. 15:245–252.

6. Birnbaum LS, Harris MW, Stocking LM, Clark AM, Morrissey RE. Retinoic acid and 2,3,7,8-tetrachlorodibenzo-p-dioxin selectively enhance teratogenesis in C57BL/6N mice. Toxicol Appl Pharmacol. 1989. 98:487–500.

7. Faqi AS, Dalsenter PR, Merker HJ, Chahoud I. Reproductive toxicity and tissue concentrations of low doses of 2,3,7,8-tetrachlorodibenzo-p-dioxin in male offspring rats exposed throughout pregnancy and lactation. Toxicol Appl Pharmacol. 1998. 150:383–392.

8. Mably TA, Moore RW, Goy RW, Peterson RE. In utero and lactational exposure of male rats to 2,3,7,8-tetrachlorodibenzo-p-dioxin. 2. Effects on sexual behavior and the regulation of luteinizing hormone secretion in adulthood. Toxicol Appl Pharmacol. 1992. 114:108–117.

9. Mably TA, Bjerke DL, Moore RW, Gendron-Fitzpatrick A, Peterson RE. In utero and lactational exposure of male rats to 2,3,7,8-tetrachlorodibenzo-p-dioxin. 3. Effects on spermatogenesis and reproductive capability. Toxicol Appl Pharmacol. 1992. 114:118–126.

10. Theobald HM, Peterson RE. In utero and lactational exposure to 2,3,7,8-tetrachlorodibenzo-rho-dioxin: effects on development of the male and female reproductive system of the mouse. Toxicol Appl Pharmacol. 1997. 145:124–135.

11. Mooradian AD, Morley JE, Korenman SG. Biological actions of androgens. Endocr Rev. 1987. 8:1–28.

12. Bjerke DL, Peterson RE. Reproductive toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin in male rats: different effects of In utero versus lactational exposure. Toxicol Appl Pharmacol. 1994. 127:241–249.

13. Ohsako S, Miyabara Y, Nishimura N, Kurosawa S, Sakaue M, Ishimura R, et al. Maternal exposure to a low dose of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) suppressed the development of reproductive organs of male rats: dose-dependent increase of mRNA levels of 5alpha-reductase type 2 in contrast to decrease of androgen receptor in the pubertal ventral prostate. Toxicol Sci. 2001. 60:132–143.
14. Ohsako S, Miyabara Y, Sakaue M, Ishimura R, Kakeyama M, Izumi H, et al. Developmental stage-specific effects of perinatal 2,3,7,8-tetrachlorodibenzo-p-dioxin exposure on reproductive organs of male rat offspring. Toxicol Sci. 2002. 66:283–292.
15. Theobald HM, Roman BL, Lin TM, Ohtani S, Chen SW, Peterson RE. 2,3,7,8-tetrachlorodibenzo-p-dioxin inhibits luminal cell differentiation and androgen responsiveness of the ventral prostate without inhibiting prostatic 5alpha-dihydrotestosterone formation or testicular androgen production in rat offspring. Toxicol Sci. 2000. 58:324–338.
16. de Kretser DM, Loveland KL, Meinhardt A, Simorangkir D, Wreford N. Spermatogenesis. Hum Reprod. 1998. 13:Suppl 1. 1–8.

17. Rodriguez I, Ody C, Araki K, Garcia I, Vassalli P. An early and massive wave of germinal cell apoptosis is required for the development of functional spermatogenesis. EMBO J. 1997. 16:2262–2270.

18. Richburg JH, Boekelheide K. Mono-(2-ethylhexyl) phthalate rapidly alters both Sertoli cell vimentin filaments and germ cell apoptosis in young rat testes. Toxicol Appl Pharmacol. 1996. 137:42–50.

19. Nandi S, Banerjee PP, Zirkin BR. Germ cell apoptosis in the testes of Sprague Dawley rats following testosterone withdrawal by ethane 1,2-dimethanesulfonate administration: relationship to Fas? Biol Reprod. 1999. 61:70–75.

20. Kim IH, Son HY, Cho SW, Ha CS, Kang BH. Zearalenone induces male germ cell apoptosis in rats. Toxicol Lett. 2003. 138:185–192.

21. Schultz R, Suominen J, Värre T, Hakovirta H, Parvinen M, Toppari J, et al. Expression of aryl hydrocarbon receptor and aryl hydrocarbon receptor nuclear translocator messenger ribonucleic acids and proteins in rat and human testis. Endocrinology. 2003. 144:767–776.

22. Bravo R. Synthesis of the nuclear protein cyclin (PCNA) and its relationship with DNA replication. Exp Cell Res. 1986. 163:287–293.

23. Bravo R, Frank R, Blundell PA, Macdonald-Bravo H. Cyclin/PCNA is the auxiliary protein of DNA polymerase-delta. Nature. 1987. 326:515–517.
24. Morris GF, Mathews MB. Regulation of proliferating cell nuclear antigen during the cell cycle. J Biol Chem. 1989. 264:13856–13864.

25. Schlatt S, Weinbauer GF. Immunohistochemical localization of proliferating cell nuclear antigen as a tool to study cell proliferation in rodent and primate testes. Int J Androl. 1994. 17:214–222.

26. Gray LE Jr, Kelce WR, Monosson E, Ostby JS, Birnbaum LS. Exposure to TCDD during development permanently alters reproductive function in male Long Evans rats and hamsters: reduced ejaculated and epididymal sperm numbers and sex accessory gland weights in offspring with normal androgenic status. Toxicol Appl Pharmacol. 1995. Mar. 131:108–118.
27. el-Sabeawy F, Wang S, Overstreet J, Miller M, Lasley B, Enan E. Treatment of rats during pubertal development with 2,3,7,8-tetrachlorodibenzo-p-dioxin alters both signaling kinase activities and epidermal growth factor receptor binding in the testis and the motility and acrosomal reaction of sperm. Toxicol Appl Pharmacol. 1998. 150:427–442.

28. Cooke GM, Price CA, Oko RJ. Effects of In utero and lactational exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) on serum androgens and steroidogenic enzyme activities in the male rat reproductive tract. J Steroid Biochem Mol Biol. 1998. 67:347–354.

29. Chang C, Saltzman A, Yeh S, Young W, Keller E, Lee HJ, et al. Androgen receptor: an overview. Crit Rev Eukaryot Gene Expr. 1995. 5:97–125.

30. McIntyre BS, Barlow NJ, Foster PM. Male rats exposed to linuron In utero exhibit permanent changes in anogenital distance, nipple retention, and epididymal malformations that result in subsequent testicular atrophy. Toxicol Sci. 2002. 65:62–70.

31. Hotchkiss AK, Parks-Saldutti LG, Ostby JS, Lambright C, Furr F, Vandenbergh JG, et al. A mixture of the "antiandrogens" linuron and butyl benzyl phthalate alters sexual differentiation of the male rat in a cumulative fashion. Biol Reprod. 2004. 71:1852–1861.

32. Scott HM, Hutchison GR, Mahood IK, Hallmark N, Welsh M, Gendt KD, et al. Role of androgens in fetal testis development and dysgenesis. Endocrinology. 2007. 148:2027–2036.

33. Hamm JT, Sparrow BR, Wolf D, Birnbaum LS. In utero and lactational exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin alters postnatal development of seminal vesicle epithelium. Toxicol Sci. 2000. 54:424–430.

34. Pirkle JL, Wolfe WH, Patterson DG, Needham LL, Michalek JE, Miner JC, et al. Estimates of the half-life of 2,3,7,8-tetrachlorodibenzo-p-dioxin in Vietnam Veterans of Operation Ranch Hand. J Toxicol Environ Health. 1989. 27:165–171.

35. Perry JE, Tindall DJ. Androgens regulate the expression of proliferating cell nuclear antigen posttranscriptionally in the human prostate cancer cell line, LNCaP. Cancer Res. 1996. 56:1539–1544.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download