Abstract
Purpose
This study was undertaken to determine the neuroprotective effect of granulocyte stimulating factor (G-CSF) on neonatal hypoxic-ischemic brain injury.
Materials and Methods
Seven-day-old male newborn rat pups were subjected to 110 minutes of 8% oxygen following a unilateral carotid artery ligation. Apoptosis was identified by performing terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling (TUNEL) staining and flow cytometry with a combination of fluorescinated annexin V and propidium iodide (PI) and JC-1 (5,5',6,6'-tetrachloro-1,1',3,3'-tetraethylbenzimidazolyl-carbocyanine iodide). The extent of cerebral infarction was evaluated at 2 weeks after recovery.
Results
With a single dose (50 µg/ kg) of G-CSF treatment immediately after hypoxic-ischemic insult, hypoxia-ischemia induced increase in TUNEL-positive cells, annexinV+/PI-and JC-1 positive apoptotic cells in the ipsilateral cerebral cortex was significantly reduced at 24 hours, measured by flow cytometry, and the extent of cerebral infarction at 2 weeks after recovery was also significantly attenuated compared to the hypoxia-ischemia control group.
Despite uninterrupted improvements in fetal monitoring and neonatal intensive care medicine, perinatal hypoxic-ischemic encephalopathy still remains an important cause of neonatal mortality and permanent neurological sequelae such as cerebral palsy, mental retardation, learning disabilities, and epilepsy.1 At present, there is no clinically efficacious treatment available for this common disorder. Therefore, the development of a new therapeutic modality to improve the prognosis of this disease is an urgent issue.
Recently, the neuroprotective effects of exogenously administered G-CSF have been reported in several adult animal models of ischemic brain injury2-8 as well as in phases I/II clinical studies of stroke.9,10 The neuroprotective effects exerted by G-CSF are known to be mediated by a multitude of mechanisms,11 including inhibition of apoptosis,6 anti-inflammatory effects,3 and stimulation of endogenous stem cell proliferation.7,8 G-CSF is one of the few growth factors that have been approved for clinical use to treat neutropenia, even in newborn infants.12,13 Therefore, any favorable experimental results can readily be translated into clinical practice. However, results obtained in adults cannot necessarily be extrapolated into neonatal medicine due to the dramatic differences in maturational stage and pathophysiology of perinatal and adult brains. Up to now, only 2 studies have reported the effects of G-CSF in neonatal brain injury. While its beneficial effects have been shown in one study,14 G-CSF was found to be detrimental to neonatal brain injury in the other.15 Therefore, the role of G-CSF in neonatal brain injury is still controversial, and further studies are necessary to clarify this.
This study was undertaken to determine whether G-CSF could reduce cerebral injury following HI in the developing brain. As we have already shown in our previous studies,16,17 apoptosis plays a major role in the development of hypoxic-ischemic neonatal brain injury. In the present study, we tested whether G-CSF is neuroprotective by inhibiting apoptosis, thereby reducing the ensuing cerebral infarction in a newborn rat pup model of cerebral HI. Apoptosis was identified by TUNEL staining and flow cytometry with a combination of fluorescinated annexin V and propidium iodide (PI) and JC-1 (5,5',6,6'-tetrachloro-1,1',3,3'-tetraethylbenzimidazolyl-carb ocyanine iodide). The extent of cerebral infarction was evaluated at 2 weeks after HI to determine whether the neuroprotective effect, if any, of G-CSF was transient or sustained.
The experimental protocols described herein were reviewed and approved by the Animal Care and Use Committee of Samsung Biomedical Research Center, Seoul, Korea. This study was performed in accordance with the institutional and National Institutes of Health guidelines for laboratory animal care. Seven-day-old Sprague-Dawley male rats (Daihan Biolink Co., Seoul, Korea) weighing 13 - 16 g were used for the experiments to exclude any gender-related differences in brain injury.18 The P7 male rat pups (n = 56) were randomly divided into 3 groups: (1) normoxia control group (NC, n = 18); (2) HI control group (HC, n = 16); and (3) HI with G-CSF treatment group (HG, n = 22). Cerebral HI was induced by a modification of the method16,17 originally reported by Rice et al.19 The neck was incised in the midline, and the right common carotid artery was permanently ligated with 4 - 0 silk under methoxyflurane anesthesia. After 2 hours of recovery period, the animals were exposed to 110 minutes of hypoxia (8% O2/92% N2) by placing them in airtight containers that had been partially submerged in a 37℃ water bath to maintain a constant thermal environment. Pups in NC received a Sham operation without carotid artery ligation. Animals in HG received an intraperitoneal injection of G-CSF (a kind gift from Donga Pharm. Co., Seoul, Korea) at a dose of 50 µg/kg of body weight immediately after the hypoxic insult,8 or an equal volume of normal saline as a NC and HC. Then, the pups were returned to their dams and sacrificed at 24 hours after HI for TUNEL stain and flow cytometry (n = 11 in NC, 7 in HC, and 11 in HG) under deep pentobarbital anesthesia (60 mg/kg, intraperitoneal). The remaining rat pups (n = 7 in NC, 9 in HC, and 11 in HG) were weighed daily for 2 weeks after HI until sacrifice for brain volume measurements.
Fresh whole brain tissue obtained at 24 hours after HI was placed on a glass petri dish on ice containing 1 - 2 mL of phosphate-buffered saline (PBS), and transected in the coronal plane at the level of the mid-dorsal hippocampus.20 The anterior portion of the right cerebral hemispheres was processed for TUNEL staining, and the posterior portion of the cerebral hemisphere within the middle cerebral artery territory, approximately 100 mg/block, was dissociated into a single cell and used for flow cytometric measurements.16,17
The extent of DNA fragmentation was delineated by analyzing brain tissue obtained from the anterior half of the ipsilateral cerebral cortex using a peroxidase in situ apoptosis detection kit S7100 (Chemicon International, Temecula, CA, USA) at 24 hours after HI. TUNEL-positive cells were identified by an optical microscope with 400 high power fields. Ten images were taken from each sample and the number of TUNEL-positive cells on a high power field was determined.
To evaluate the extent of apoptotic and necrotic cells, the posterior portion of the ipsilateral cerebral cortex was dissociated into a single cell and flow cytometry was done with a combination of PI (Sigma, St. Louis, MO, USA) and annexin V-FITC (fluorescein isothiocyanate) (Pharmingen, San Diego, CA, USA). Flow cytometric analysis was performed by PAS (Particle Analyzing System, Partec, Münster, Germany) equipped with an argon ion laser tuned at 488 nm wavelength. Green FITC-annexin V fluorescence was measured at 530 ± 15 nm, and red PI fluorescence was measured at 600 nm.16,17
Mitochondrial membrane potential was estimated using fluorescent probe JC-1 (Molecular Probes, Eugene, OR, USA).21 The dissociated cortical cell suspensions were adjusted to the density of 1 × 106 cells/mL and stained for 20 minutes with 2.0 µg/mL of JC-1 at 37℃. JC-1 was excited with the 488 nm argon laser, and JC-1 green and orange fluorescences were recorded on FL1 (530 ± 15 nm band pass filter) and FL2 (575 ± 13 nm band pass filter) channels.
After transcardiac perfusion with 0.1 M of PBS, the brains were carefully removed at 2 weeks after HI and blocked with paraffin after overnight fixation with 4% paraformaldehyde. The 5 µm thick serial sections were made at an interval of 100 µm and stained with hematoxylin-eosin for volumetric analysis.
All volumetric qualifications were performed with an Olympus BX 40 photomicroscope (Olympus Optical Co., Ltd., Tokyo, Japan) equipped with a high resolution CCD camera, a motorized XYZ axis computer-controlled stage, and Stereoinvestigator software package (ver. 6.52, Micro Bright Field, VT, USA). When calculating volume, the cross-sectional areas of the region of interest (ROI) in each section were traced on the computer screen at low power with a 1.5 ×/4 × lens, and volume of the ROI was calculated by using the Stereoinvestigator software according to Cavalieri's principle.22
Using this sampling strategy, approximately 8 histology sections per brain in the affected and control hemisphere in each animal were evaluated for hemispheric measurements. For anatomical evaluation of the extent of cerebral injury, the ratio of the ipsilateral remaining cerebral hemisphere volume to the volume of the corresponding contralateral cerebral hemisphere is expressed as a percentage. An examiner blind to the treatment group quantified.
All data are expressed as mean ± standard deviation. Kruskal-Wallis test was used for multiple group comparisons followed by Wilcoxon rank sum test including Bonferroni adjustment for a comparison between 2 groups. All statistical analyses described above were carried out using the SAS Enterprise Guide version 3 (SAS Institute, Cary, NC, USA). A p value of < 0.05 was considered significant.
In the ipsilateral anterior portion of the cerebral cortex at 24 hours after HI significantly increased numbers of TUNEL-positive cells were observed in HC compared to NC (59.6 ± 9.2 vs. 0.2 ± 0.1, p < 0.01). However, this increase in the number of apoptotic cells was significantly attenuated in HG compared to HC (14.3 ± 16.9 vs. 59.6 ± 9.2, p < 0.05) (Fig. 1).
Representative analyses and regional percentage of an annexin V versus PI dot plot of the ipsilateral cerebral cortex in NC, HC, and HG at 24 hours after HI are presented in Fig. 2A. In HG, the increased percentage of apoptotic cells in Q4 (annexin V+/PI-) and decreased percentage of live cells in Q3 (annexin V-/PI-) observed in HC were significantly improved but the attenuation of secondary necrotic cells in Q2 (annexin V+/PI+) did not reach statistical significance, and the damaged cells in Q1 (annexin V-/PI+) were not significantly different from those in HC (Table 1).
Significantly retarded body weight gain was observed in HC compared to NC, starting from 3 days after HI (p < 0.05). The weight gain, which is indicative of general well being, was significantly improved in HG from 10 days after HI compared to HC (p < 0.05) (Fig. 3).
In HC, there was a significant decrease in per centage of intact ipsilateral hemispheric volume compared to NC (p < 0.01) at 2 weeks after HI injury. The HG group showed a marked protective effect and significantly increased percentage of ipsilateral hemispheric volume compared to the HC group (p < 0.01) 2 weeks after HI (Fig. 4).
In the present study, G-CSF significantly attenuated the HI-induced loss of body weight and increased apoptosis and ensuing cerebral infarction in the newborn rat. In accordance with our data, Yata et al.14 have also reported the neuroprotective effects of G-CSF in neonatal-hypoxic ischemic brain injury. The safety and feasibility of G-CSF have been demonstrated in recent human stroke therapy trials.9,10 Furthermore, G-CSF is already available clinically in neonates.12,13 Taken together, these findings strongly support the potential use of G-CSF as therapeutic agents in the clinical setting of perinatal hypoxic ischemic encephalopathy where effective treatments have not yet been established.
Although the mechanisms by which G-CSF serves a neuroprotective agent have not fully been clarified, G-CSF may act in a coordinated fashion at multiple levels,11 including inhibition of apoptosis,6 modulation of inflammation,3 and recruitment of endogenous stem cells.7,8 In the present study, TUNEL staining was performed to detect nuclear DNA breakdown, a common feature of apoptosis.23 Flow cytometry using annexin V was done to detect exposure of phosphatidylserine outside of the plasma membrane, the early and most characteristic change of apoptosis.24,25 PI was used to detect the disruption of the plasma membrane and alterations in permeability observed in necrotic cells. Decreased mitochondrial membrane potential, observed during early apoptosis, were measured by counting the shift of JC-1 fluorescence from orange to green with flow cytometry.21 Together, our data on HI-induced increase in TUNEL-positive cells, apoptotic (annexin V+/PI-) cells, and JC-1 fluorescence shifted from orange to green support the assumption that apoptosis is the primary mode of cell death following HI in the developing brain.16,17,26 Our data of significant attenuation of these abnormalities with G-CSF treatment also support the assumption that the neuroprotective action of G-CSF is mediated mainly by inhibition of apoptosis in a newborn rat model of cerebral HI.14
In the present study, a single dose of G-CSF given immediately after HI significantly attenuated the cerebral infarction at 2 weeks after HI. These results implicate that the neuroprotective effects of G-CSF are not transient. In our previous studies,16,17 we have shown that apoptosis plays an important role in the development of delayed infarction, and that the inhibition of apoptosis with cycloheximide significantly attenuates ensuing cerebral infarction in the newborn rat model of cerebral HI. Therefore, our data on significant reduction of infarction with G-CSF treatment strongly suggest that G-CSF given immediately after HI could attenuate and prevent the activation of subsequent neurotoxic, probably apoptotic cascade, ultimately leading to irreversible neuronal cell death and delayed cerebral infarction occurring days or months later.26-29
In the present study, G-CSF was given immediately after HI. However, as most asphyxial events occur before birth and enrollment of newborns for clinical trials can take at least several hours for a mother to recover from a delivery and to give informed consent, it is practically impossible to start any therapy immediately after birth. There is a danger that delay in applying potentially brain-saving treatments beyond the therapeutic window might abolish or drastically reduce its therapeutic effectiveness.17,27-29 Therefore, the long therapeutic window of G-CSF reported in both preclinical8 and clinical studies of adult stroke9,10 suggests therapeutic potential of this agent in humans with perinatal hypoxic-ischemic encephalopathy. Further studies on dosage, therapeutic window, and safety in neonates are necessary for the successful translation of the benefits of G-CSF treatment observed in this animal study into clinical trials.
In summary, G-CSF was demonstrated to have neuroprotective effects by inhibiting apoptosis, reducing the ensuing cerebral infarction in a newborn rat pup model of cerebral HI. Our data suggest that G-CSF is a potentially novel therapeutic agent in perinatal hypoxic-ischemic encephalopathy, and further clinical studies are warranted.
Figures and Tables
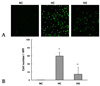 | Fig. 1Representative photomicrographs of TUNEL staining (A) at the anterior portion of the ipsilateral cerebral cortex (× 400) and number of TUNEL-positive cells (B) per high power field in the NC, HC, and HG groups at 24 hours after HI. Note significantly increased TUNEL-positive green cells in HC compared to NC and less TUNEL-positive cells in HG compared to HC. All data are mean ± standard deviation. *p < 0.05 compared to NC. †p < 0.05 compared to HC. NC, normoxia control group; HC, HI control group; HG, HI with G-CSF treatment group. |
 | Fig. 2(A) Representative flow cytogram of an annexin V binding (abscissa) versus PI uptake (ordinate) in the ipsilateral cerebral hemisphere of newborn rat brain cells at 24 hours after HI. The numbers in the upper left quadrant (Q1), upper right quadrant (Q2), lower left quadrant (Q3), and lower right quadrant (Q4) represent the percentage of damaged (annexin V-/PI+), necrotic (annexin V+/PI+), live (annexin V-/PI-) and apoptotic cells (annexin V+/PI-), respectively. (B) Representative flow cytometric analyses of mitochondrial membrane potential using JC-1. The shift of JC-1 fluorescence from orange (FL2) to green (FL1) indicates a collapse of mitochondrial membrane potential. NC, normoxia control group; HC, HI control group; HG, HI with G-CSF treatment group. |
 | Fig. 3Body weight gain, indicative of general well being, in the rat pups of each experimental group. Significantly less body weight gain was observed in HC compared to NC staring from 3 days after HI, and was improved in HG from 10 days after HI compared to HC. NC, normoxia control group; HC, HI control group; HG, HI with G-CSF treatment group. All data are mean ± standard deviation. *p < 0.05 compared to NC. †p < 0.05 compared to HC. |
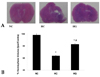 | Fig. 4Representative photomicrographs of hematoxylin/eosin staining (A) at the anterior commisure and percentage of the preserved brain volume (B) from the normoxia control (NC), HI control (HC), and HI with G-CSF treatment (HG) groups at 2 weeks after the injury. Significantly increased infarct volume in HC compared to NC, and significantly reduced infarct volume of HG compared to HC are observed. NC, normoxia control group; HC, HI control group; HG, HI with G-CSF treatment group. All values are mean ± standard deviation. *p < 0.05 compared to NC. †p < 0.05 compared to HC. |
Table 1
Regional Cell Percentage of Ipsilateral Cerebral Hemisphere at 24 Hours after Hypoxia-ischemia

Q1, damaged (annexinV-/PI+) cells; Q2, necrotic (annexinV+/PI+) cells; Q3, live (annexinV-/PI-) cells; Q4, apoptotic (annexinV+/PI-) cells; NC, normoxia control group; HC, HI control group; HG, HI with G-CSF treatment group. All values given are mean ± standard deviation.
*p < 0.05 compared to NC.
†p < 0.05 compared to HC.
References
2. Gibson CL, Bath PM, Murphy SP. G-CSF reduces infarct volume and improves functional outcome after transient focal cerebral ischemia in mice. J Cereb Blood Flow Metab. 2005. 25:431–439.

3. Gibson CL, Jones NC, Prior MJ, Bath PM, Murphy SP. G-CSF suppresses edema formation and reduces interleukin-1beta expression after cerebral ischemia in mice. J Neuropathol Exp Neurol. 2005. 64:763–769.

4. Lee ST, Chu K, Jung KH, Ko SY, Kim EH, Sinn DI, et al. Granulocyte colony-stimulating factor enhances angiogenesis after focal cerebral ischemia. Brain Res. 2005. 1058:120–128.

5. Schäbitz WR, Kollmar R, Schwaninger M, Juettler E, Bardutzky J, Schölzke MN, et al. Neuroprotective effect of granulocyte colony-stimulating factor after focal cerebral ischemia. Stroke. 2003. 34:745–751.

6. Schneider A, Krüger C, Steigleder T, Weber D, Pitzer C, Laage R, et al. The hematopoietic factor G-CSF is a neuronal ligand that counteracts programmed cell death and drives neurogenesis. J Clin Invest. 2005. 115:2083–2098.

7. Shyu WC, Lin SZ, Yang HI, Tzeng YS, Pang CY, Yen PS, et al. Functional recovery of stroke rats induced by granulocyte colony-stimulating factor-stimulated stem cells. Circulation. 2004. 110:1847–1854.

8. Six I, Gasan G, Mura E, Bordet R. Beneficial effect of pharmacological mobilization of bone marrow in experimental cerebral ischemia. Eur J Pharmacol. 2003. 458:327–328.
9. Shyu WC, Lin SZ, Lee CC, Liu DD, Li H. Granulocyte colony-stimulating factor for acute ischemic stroke: a randomized controlled trial. CMAJ. 2006. 174:927–933.

10. Schabitz WR, Schneider A. Developing granulocyte-colony stimulating factor for the treatment of stroke: current status of clinical trials. Stroke. 2006. 37:1654.
11. Solaroglu I, Cahill J, Jadhav V, Zhang JH. A novel neuroprotectant granulocyte-colony stimulating factor. Stroke. 2006. 37:1123–1128.
12. Ahmad M, Fleit HB, Golightly MG, La Gamma EF. In vivo effect of recombinant human granulocyte colony-stimulating factor on phagocytic function and oxidative burst activity in septic neutropenic neonates. Biol Neonate. 2004. 86:48–54.

13. Carr R, Modi N. Haemopoietic growth factors for neonates: assessing risks and benefits. Acta Paediatr Suppl. 2004. 93:15–19.

14. Yata K, Matchett GA, Tsubokawa T, Tang J, Kanamaru K, Zhang JH. Granulocyte-colony stimulating factor inhibits apoptotic neuron loss after neonatal hypoxia-ischemia in rats. Brain Res. 2007. 1145:227–238.

15. Keller M, Simbruner G, Górna A, Urbanek M, Tinhofer I, Griesmaier E, et al. Systemic application of granulocyte-colony stimulating factor and stem cell factor exacerbates excitotoxic brain injury in newborn mice. Pediatr Res. 2006. 59(4 Pt 1):549–553.

16. Park WS, Sung DK, Kang S, Koo SH, Kim YJ, Lee JH, et al. Neuroprotective effect of cycloheximide on hypoxic-ischemic brain injury in neonatal rats. J Korean Med Sci. 2006. 21:337–341.
17. Park WS, Sung DK, Kang S, Koo SH, Kim YJ, Lee JH, et al. Therapeutic window for cycloheximide treatment after hypoxic-ischemic brain injury in neonatal rats. J Korean Med Sci. 2006. 21:490–494.

18. Zhu C, Xu F, Wang X, Shibata M, Uchiyama Y, Blomgren K, et al. Different apoptotic mechanisms are activated in male and female brains after neonatal hypoxia-ischaemia. J Neurochem. 2006. 96:1016–1027.
19. Rice JE 3rd, Vannucci RC, Brierley JB. The influence of immaturity on hypoxic ischemic brain damage in the rat. Ann Neurol. 1981. 9:131–141.
20. Vannucci RC, Towfighi J, Vannucci SJ. Secondary energy failure after cerebral hypoxia-ischemia in the immature rat. J Cereb Blood Flow Metab. 2004. 24:1090–1097.

21. Zuliani T, Duval R, Jayat C, Schnébert S, André P, Dumas M, et al. Sensitive and reliable JC-1 and TOTO-3 double staining to assess mitochondrial transmembrane potential and plasma membrane integrity: interest for cell death investigations. Cytometry A. 2003. 54:100–108.

22. Regeur L, Pakkenberg B. Optimizing sampling designs for volume measurements of components of human brain using a stereological method. J Microsc. 1989. 155:113–121.

23. Renvoizé C, Biola A, Pallardy M, Bréard J. Apoptosis: identification of dying cells. Cell Biol Toxicol. 1998. 14:111–120.
24. Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J Immunol Methods. 1995. 184:39–51.

25. Honda O, Kuroda M, Joja I, Asaumi J, Takeda Y, Akaki S, et al. Assessment of secondary necrosis of Jurkat cells using a new microscopic system and double staining method with annexin V and propidium iodide. Int J Oncol. 2000. 16:283–288.

26. Zhu C, Wang X, Xu F, Bahr BA, Shibata M, Uchiyama Y, et al. The influence of age on apoptotic and other mechanisms of cell death after cerebral hypoxia-ischemia. Cell Death Differ. 2005. 12:162–176.

27. Linnik MD, Zobrist RH, Hatfield MD. Evidence supporting a role for programmed cell death in focal cerebral ischemia in rats. Stroke. 1993. 24:2002–2008. discussion 2008-9.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download