Abstract
Purpose
Melatonin, the most potent scavenger of toxic free radicals, has been found to be effective in protecting against pathological states due to the release of reactive oxygen species. This study was performed to establish the effect of high dose melatonin on protection against ischemia-reperfusion (I/R) injury in rat hearts.
Materials and Methods
Forty male Sprague-Dawley rats were used in this study. They were separated into four groups of ten rats each. A left coronary artery occlusion was induced in the rats by ligating the artery for 20 minutes and then releasing the ligation (reperfusion) afterwards. The control group was Group A. Group B was subjected to myocardial ischemia-reperfusion without any treatment, while Group C underwent myocardial ischemia-reperfusion with a melatonin treatment before the ischemia. Group D was subjected to myocardial ischemia-reperfusion with a melatonin treatment before the reperfusion. After 20 minutes of reperfusion, blood samples were obtained from each group for biochemical studies, and the animals were sacrificed for histological and, immunohistochemical examinations of the myocardial tissue.
Results
We found that the cardiac troponin T(cTn-T) levels were significantly increased in Group B when all groups were compared. In the Group C rats treated with melatonin, the cTn-T values were significantly lower than those in Groups B and D. In addition, malondialdehyde (MDA) and antioxidant enzymes including, superoxide dismutase (SOD) and myeloperoxidase (MPO) were lower than those in Group B in the melatonin treated groups. The differences were statistically significant (p < 0.05). Histopathologic and immunohistopathologic studies also supported the effectiveness of melatonin.
Cell injury occurring after ischemia-reperfusion (I/R) in the heart is attributed to necrosis caused by calcium overload, acidosis, and oxidative stress.1 After I/R, myocardial cells die by necrosis and/or apoptosis. Apoptosis is a process for disposing of injured or redundant cells through self-destruction.2 Free radicals and antioxidants play an important role in cellular damage that can cause atherosclerosis and myocardial infarction. Both neutrophils and free radicals, such as superoxide anions, hydroxyl radicals and hydrogen peroxide, can cause oxidative damage to cell lipids, proteins, and nucleic acids.3
Melatonin (N-acetyl-5-methoxytryptamine) is secreted by the pineal gland, and has been confirmed to be potent scavenger of hydroxyl and peroxyl radicals.3,4 Many researchers have considered the antioxidant role of melatonin and it is ability to trap cellular free radicals.5 In addition, melatonin has also been shown to act as an immune system modulator.6 Although there is a large amount of consistent data available that shows the potential protective effect of melatonin in cells, a consensus still exists that reactive oxygen species play an important role in the pathogenesis of cardiac reperfusion injury.7 Several previous studies have examined melatonin and its effect on cell death following ischemia-reperfusion. What differentiates our study is the combination of histological parameters, immunohistopathologic studies and biochemical parameters that support the hypothesis that high-dose melatonin provides effective protection following I/R.
This study was performed in the Hakan Çetinsaya Clinical and Experimental-Research Center at Erciyes University. The Ethics Committee approved this study. All animals involved received humane care in compliance with the European Convention on Animal Care.
Male Sprague-Dawley rats (n = 40) weighing 310 to 340 g (average 321 g) were used in this study. The rats were anesthetized with ketamine hydrochloride (20 mg/kg), intraperitoneally (i.p.), and heparin (500 IU/kg, i.p) was also administered.
A left thoracotomy was performed, and the pericardium was incised. The left coronary artery was surgically occluded through ligation with a suture (monoflamant polypropylene, size 6.0) and than followed with a coronary reperfusion through the release of the tie.
Group A (n = 10) was the control group and no procedures were carried out.
Group B (n = 10) was subjected to cardiac ischemia (20 minutes) - reperfusion (20 minutes) without any treatment.
Group C (n = 10) was treated with melatonin (50 mg/kg i.p, Sigma, M-5250, St. Louis, MO, USA) 30 minutes before ischemia (20 minutes) - reperfusion (20 minutes).
Group D (n = 10) uderwent left coronary artery occlusion (ischemia time of 20 minutes) and then melatonin (50 mg/kg i.p.) was administered just before reperfusion.
Rats that developed ventricular fibrillation or cardiac arrest during I/R were excluded from the study, so only the 40 rats that did not have any complications were used. For biochemical testing blood samples were taken from all rats via the atrium using a 22 G intravenous cannula (Polyflon) after 20 minutes reperfusion. Heparinized blood obtained from the rats was centrifuged at 2,000 rpm for 15 minutes at 4℃. After separating the plasma, the samples were stored at -20℃ until analysis. Tissue samples were taken from the left ventricle and bathed in a 10% formalin solution for pathologic study. The tissue samples were stained with Hematoxylin and Eosin (H & E) and were evaluated by light microscopy.
Over the last few years, compelling evidence has established an important physiologic role for apoptotic cell death in maintaining optimal cell numbers in multicellular organisms. Proteins such as Bcl-2 or Fas and its ligand, have been shown to regulate this process.
Representative sections of the heart tissue were fixed in 10% neutral buffered formalin for 24 hours. After standard histological processing the sections were embedded in paraffin, then 5-µm paraffin sections were deparaffinized with three rinses of xylene, and than followed by rehyded with ethanol. To unmask any antigenic determinants, slides were pretreated in a microwave for 10 minutes in a 0.01 M. citrate buffer, treated for 30 minutes in 0.5% H2O2, then washed with PBS (phosphate buffered saline: SIGMA #1000-3). Next the sections were incubated with the primary antibodies (1 : 10 dilution overnight for Fas, 1 : 50 dilution overnight for Bcl-2). Both mouse monoclonal antibodies against Fas, and mouse monoclonal antibodies against Bcl-2 (both from DAKO Corporation, Carpenteria, CA, USA) were used in this study. The immunohistochemical staining was performed using the streptoavidin-biotin kit (DAKO Corporation, Carpenteria, CA, USA) by the avidin-biotin-peroxidase method, according to the manufacturer's instructions. The peroxidase reaction was developed with 3,3'-diaminobenzidine, and the slides were counterstained with hematoxylin. Formalin-fixed, paraffin-embedded sections of the colon were used as positive controls for Fas, and tonsil sections were used as controls in Bcl-2 staining.
The intensity of immunostaining was evaluated by repeated staining of the same specimens, and an observer who had no knowledge of the experimental group examined the staining. The immunostaining was graded as "0" for no immunostaining or as, "1" if immunostaining was definitely detectable.
The assay mixture was composed of a 0.3 mL 100 mM phosphate buffer (pH 6.0; 0.3 mL 10 mM H2O2, 0.5 mL 20 mM o-dionisidine (freshly prepared) in deionized water; and 10 mL PMNL homogenate in a final volume of 3.0 mL. The absorbance at 460 nm was followed for 10 minutes. All measurements were carried out in duplicate. One unit of MPO was defined as that which degrade 1 mmol H2O2/min at 25℃; specific activity was given as U/mg of protein. A molar extinction coefficient of 11,300 for oxidized o-dianisidine was used for the calculation.
The determination of MDA, was measured using the spectrophotometrical method defined by Ohkawa et al. in 1979. MDA couples to thiobarbituric acid to form a pink chromogen compound, which has a maximum absorbance at 540 nm in wavelength. Plasma MDA levels were expressed as micromoles per liter (µmol MDA/liter).
The enzymatic activity of SOD was measured according to the inhibition of nitroblue tetrazolium (NBT) reduction with xanthine-xanthine oxidase used as a superoxide generator. One unit of SOD activity was defined as the amount of protein that inhibited the rate of NBT reduction by 50%. Enzyme activity was expressed as U/mg protein.
In Group A, cTn-T values were significantly lower in comparison with all other groups. The cTn-T values in Group B were significantly higher compared with all other groups. In Group C, where melatonin treatment was used before ischemia, cTn-T values were significantly lower than Groups B and D, which signified the efficacy of pre-ischemia treatment (Table 1).
The values of MDA in Group A, were significantly lower compared with all groups. In both Groups C and D where melatonin treatments were used a significantly lower level of MDAs was seen than that of Group B (Table 1).
The values of MPO in both Groups C and D, which received melatonin treatments, were significantly lower than in Group B (Table 1).
The values of SOD in Groups C and D, where melatonin treatments, were used showed significantly lower levels than Group B. There were no significant differences between Groups C and A for these levels (Table 1).
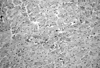
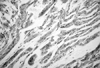
Immunohistochemical studies showed that apoptotic activity in Group B was more intensive than in the other groups (Fig. 1). Melatonin treatment was associated with diminishing Fas expression in ischemic-reperfused hearts in rats. Bcl-2 expression was significantly higher in Groups C and D (Figs 1 and 2).
It is well known that after myocardial ischemia, free radicals, cytokines and antioxidants play a major role in myocardial damage. Early reperfusion of the ischemia can save the myocardium before it becomes irreversibly injured. A delay in reperfusion often results in injury to the myocardial cells, which has been termed reperfusion injury.8,9 Oxygen-derived free radicals can cause damage to various biological targets, such as proteins, DNA, and lipids, and can also inflict severe membrane damage with consequent cell derangement or death.10 SOD is a metalloenzyme that catalyzes the dismutation of O2- into O2 and H2O2, and affords protection against free radical damage. It can also be associated with increased oxidative stress.3,11 Other important antioxidative enzymes such as MPO and MDA also can result in an increase in ischemia-reperfusion injuries.12 Troponin T is one of the regulatory proteins of the troponin complex, and cardiac troponin is one of the isoforms of this complex. Even minor myocardial damage can be detected using cardiac troponin T levels. The importance of cTn-T is its reilablity in identifying perioperative MI and in the assessment of reperfusion therapy of an infarct zone after MI.13 In our study, the levels of cTn-T, SOD, MDA, and MPO were significantly high only in the non-treatment I/R group.
Cell death that occurs after I/R in the heart is caused by energy depletion, acidosis, and oxidative stress. Apoptosis occurs during I/R, and it is a significant contributor to myocardial cell death. Death receptors are cell surface receptors that transmit apoptosis signals initiated by specific ligands. The best characterization of the death receptors is Fas. The Bcl-2 proteins are a family of proteins involved in the response to apoptosis. Some of these proteins (such as Bcl-2 and Bcl-XL) are anti-apoptotic. The sensitivity of cells to apoptotic stimuli can depend on the balance of pro- and anti-apoptotic Bcl-2 proteins. The pro-apoptotic Bcl-2 proteins are often found in the cytosol, where they act as sensors of cellular damage or stress.2,8,14 In our study, apoptotic activity was intensive in the non-treated I/R group.
Despite the discovery of melatonin more than 40 years ago, it was not recognized as an antioxidant and a free radical scavenger until the last ten years. Several investigators have shown that melatonin is a derivative of the amino-acid tryptophan, and is produced in the pineal gland. Melatonin is a free radical scavenger that has the ability to neutralize the toxicity of the hydroxyl radical, the singlet oxygen, and possibly the peroxyl radical and the superoxide anion.15 Ianas and colleagues were the first to investigate melatonin as an antioxidant.16 Later, Tan et al. demonstrated that melatonin detoxifies the hydroxyl radical.4 Sainz et al. were the first to find that both naturally-occurring and induced-apoptosis is slowed down by melatonin.17
As an antioxidant, melatonin is effective in protecting DNA, membrane lipids and, presumably, cytosolic proteins from oxidative damage. In addition to its direct free radical scavenging and membrane stabilization, melatonin has been reported to alter the activities of enzymes that improve the total antioxidative defense capacity of the organism (including SOD, and nitric oxide synthase).18
The effect of melatonin on myocardial reperfusion injury has only been studied in recent years. These studies have shown the effect of melatonin on the incidence of myocardial reperfusion arrhythmias, stunning, and the limitation of infarct size.3,19 The effect of melatonin on ischemia-reperfusion-induced arrhythmias in the isolated rat heart model has been reported, and it has been found that melatonin is a potent agent in protecting against ischemia-reperfusion injury. This is due to the hydroxyl radical scavenging effect of melatonin and also due to the reduction of the extent of lipid peroxidation.3 In reviewing studies that investigated the effects of antioxidants on the limitation of infarct size, Bolli found that approximately half of the 40 studies published between 1984 - 1989 reported a reduction in infarct size, while the other half did not find any effect. The only consistent findings were that the antioxidant had to be present during the entire reperfusion period in order to produce positive results.20 The ability of melatonin to act as a peroxyl radical scavenger was compared with that of glutathione and Vitamins E, and C. Melatonin's scavenging ability was about two times higher than that of Vitamins E, and C and almost three times higher than that of glutathione.21
The protective effect of melatonin against oxidative stress during reperfusion of the liver, lungs, intestines and brain after induced ischemia was examined using both biochemical and morphologic measurements22-25 The authors reported that exogenously administered melatonin effectively protected the liver, lungs, intestines and brain against oxidative damage. This protection was evident by reduced lipid peroxidation, lowered polymorphonuclear leukocyte infiltration, and reduced antioxidant enzymes.
The observation by Szarszoi et al. that the exogenous administration of a low physiological dose of melatonin does not provide protection does not automatically imply that melatonin is inactive at physiological concentrations.20
In our study, we demonstrated that high doses of melatonin have statistically significant effects on ischemia-reperfusion-induced myocardial injury in rat hearts. The levels of cardiac troponin T and MDA, MPO, and SOD activity in the non-treated I/R groups were higher than in those treated with high doses of melatonin. In the melatonin-treated groups both before ischemia and reperfusion, these values were significantly lower than in the non-treated group. These values were slightly lower in Group C, in which melatonin was given before ischemia, when compared to Group D, in which melatonin was administered before reperfusion. The effects of melatonin on the expression of Fas and Bcl-2 in the myocardium were determined based on the established death-promoting effect of Fas and the anti-apoptotic effect of Bcl-2. The high intensity of Bcl-2 in the melatonin treatment groups (particularly before ischemia) indicated that anti-apoptotic activity was present. All these biochemical results were supported by immunohistochemical and histopathological findings.
Although low-dose melatonin studies seem to have been inconclusive, our study has emphasized that high-dose melatonin treatment increases antioxidant enzymes and anti-apoptotic activity. In conclusion, our study confirmed that high-dose melatonin treatment acts as a protective agent against cardiac I/R in rats.
Figures and Tables
Fig. 1
Melatonin treatment was associated with decrease Fas expression while Bcl-2 expression were significantly higher in ischemic-reperfused hearts in rats Groups C and D.

References
1. Hearse DJ, Bolli R. Reperfusion induced injury: manifestations, mechanisms, and clinical relevance. Cardiovasc Res. 1992. 26:101–108.

2. Yue TL, Ma XL, Wang X, Romanic AM, Liu GL, Louden C, et al. Possible involvement of stress-activated protein kinase signaling pathway and Fas receptor expression in prevention of ischemia/reperfusion-induced cardiomyocyte apoptosis by carvedilol. Circ Res. 1998. 82:166–174.

3. Kaneko S, Okumura K, Numaguchi Y, Matsui H, Murase K, Mokuno S, et al. Melatonin scavenges hydroxyl radical and protects isolated rat hearts from ischemic reperfusion injury. Life Sci. 2000. 67:101–112.

4. Tan DX, Chen LD, Poeggeler B, Manchester LC, Reiter RJ. Melatonin: a potent, endogenous hydroxyl radical scavenger. Endocr J. 1993. 1:57–60.
5. Reiter RJ, Poeggeler B, Tan DX, Chen LD, Manchester LC, Cuerrero JM. Antioxidant capacity of melatonin: a novel action not requiring receptor. Neuro Endocrinol Lett. 1993. 15:103–116.
6. Fjaerli O, Lund T, Osterud B. The effect of melatonin on cellular activation processes in human blood. J Pineal Res. 1999. 26:50–55.

7. Hearse DJ, Tosaki A. Reperfusion-induced arrhythmias and free radicals: studies in the rat heart with DMPO. J Cardiovasc Pharmacol. 1987. 9:641–650.
8. Jeremias I, Kupatt C, Martin-Villalba A, Habazettl H, Schenkel J, Boekstegers P, et al. Involvement of CD95/Apo1/Fas in cell death after myocardial ischemia. Circulation. 2000. 102:915–920.

9. Salie R, Harper I, Cillie C, Genade S, Huisamen B, Moolman J, et al. Melatonin protects against ischaemic-reperfusion myocardial damage. J Mol Cell Cardiol. 2001. 33:343–357.

11. Rodríguez AB, Nogales G, Ortega E, Barriga C. Melatonin controls superoxide anion level: Modulation of superoxide dismutase activity in ring dove heterophils. J Pineal Res. 1998. 24:9–14.

12. Cuzzocrea S, Costantino G, Mazzon E, Micali A, De Sarro A, Caputi AP. Beneficial effects of melatonin in a rat model of splanchnic artery occlusion and reperfusion. J Pineal Res. 2000. 28:52–63.

13. Haider KH, Stimson WH. Cardiac myofibrillar proteins: biochemical markers to estimate myocardial injury. Mol Cell Biochem. 1999. 194:31–39.
14. Ohno M, Takemura G, Ohno A, Misao J, Hayakawa Y, Minatoguchi S, et al. "Apoptotic" myocytes in infarct area in rabbit hearts may be oncotic myocytes with DNA fragmentation: analysis by immunogold electron microscopy combined with in situ nick end-labeling. Circulation. 1998. 98:1422–1430.

15. Reiter R, Tang L, Garcia JJ, Muñoz-Hoyos A. Pharmacological actions of melatonin in oxygen radical pathophysiology. Life Sci. 1997. 60:2255–2271.

16. Ianăs O, Olinescu R, Bădescu I. Melatonin involvement in oxidative processes. Endocrinologie. 1991. 29:147–153.
17. Sainz RM, Mayo JC, Uría H, Kotler M, Antolín I, Rodriguez C, et al. The pineal neurohormone melatonin prevents in vivo and in vitro apoptosis in thymocytes. J Pineal Res. 1995. 19:178–188.

18. Bettahi I, Pozo D, Osuna C, Reiter RJ, Acuña-Castroviejo D, Guerrero JM. Melatonin reduces nitric oxide synthase activity in rat hypothalamus. J Pineal Res. 1996. 20:205–210.

19. Szárszoi O, Asemu G, Vanecek J, Ost'ádal B, Kolár F. Effects of melatonin on ischemia and reperfusion injury of the rat heart. Cardiovasc Drugs Ther. 2001. 15:251–257.
20. Bolli R. Oxygen-derived free radicals and myocardial reperfusion injury: an overview. Cardiovasc Drugs Ther. 1991. 5:Suppl 2. 249–268.

21. Pieri C, Marra M, Moroni F, Recchioni R, Marcheselli F. Melatonin: a peroxyl radical scavenger more effective than vitamin E. Life Sci. 1994. 55:PL271–PL276.

22. Sewerynek E, Reiter RJ, Melchiorri D, Ortiz GG, Lewinski A. Oxidative damage in the liver induced by ischemia-reperfusion: protection by melatonin. Hepatogastroenterology. 1996. 43:898–905.
23. Inci I, Inci D, Dutly A, Boehler A, Weder W. Melatonin attenuates posttransplant lung ischemia-reperfusion injury. Ann Thorac Surg. 2000. 73:220–225.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download