Abstract
Purpose
Anaplastic large cell lymphoma (ALCL), a CD30+ T-cell non-Hodgkin's lymphoma, represents only 2 - 8% of lymphoma overall. Information on the clinical findings of primary systemic ALCL in Korea is limited. Our aims were to report the clinical features and outcomes of primary systemic ALCL.
Patients and Methods
We retrospectively reviewed the medical records of 36 adult patients diagnosed with primary systemic ALCL at Asan Medical Center from February 1995 through June 2006.
Results
Of 36 patients, 29 were male. The median age was 39 years (range, 17 - 67 years), and 26 (72%) presented with Ann Arbor stages III and IV. The most commonly involved extranodal sites were bone (n = 7) and soft tissue (n = 6). Thirty-two of all patients (89%) were treated with an anthracycline-based regimen including cyclophosphamide/doxorubicin/vincristine/prednisone (CHOP) as induction chemotherapy; 16 (50%) achieved complete remission (CR), and 13 (41%) achieved partial remission (PR). Median overall survival (OS) and event-free survival (EFS) were 49 and 17 months, respectively. Univariate analysis showed that performance status (p = 0.035), international prognostic index (IPI) (p = 0.025), and age-adjusted IPI (p = 0.034) were significant prognostic factors for OS, whereas anaplastic lymphoma kinase (ALK) expression did not affect OS (p = 0.483).
Anaplastic large cell lymphoma (ALCL) is a CD30-positive neoplasm of T-cell or null-cell lineage with characteristic clinicopathologic features, and accounts for 2 - 8% of all lymphomas.1 Two subsets of systemic ALCL are currently recognized in the World Health Organization (WHO) classification scheme.2 One subset expresses the oncogenic protein anaplastic lymphoma kinase (ALK) as a result of either the t(2;5)(p23;q35) translocation or variant alk rearrangement that does not involve npm. The second subset includes ALK-negative (ALK ) tumors that are morphologically similar to ALK-positive (ALK+) ALCL and also express CD30.3-8
The major subgroups of primary ALCL also have been distinguished by the WHO classification2 as (1) systemic ALCL, a heterogeneous subgroup with a broad spectrum of morphology, immunophenotype, and clinical characteristics; and (2) cutaneous ALCL, a spectrum of cutaneous CD30+ lymphoproliferative disorders with a uniformly good prognosis.9 The treatment of primary cutaneous ALCL is conservative, consisting of resection, with or without irradiation or low-dose methotrexate, which is used in multifocal disease not amenable to localized therapy. In contrast, primary systemic ALCL requires multiagent chemotherapy.10
Despite recent advances in the characterization of ALCL, little is known regarding the clinical characteristics of Korean adult patients with primary systemic ALCL. We therefore analyzed the clinical features and treatment outcomes of 36 adult patients with primary systemic ALCL in Korea and the clinical factors associated with prognosis.
A survey of the medical records at Asan Medical Center (Seoul, Korea) identified 65 patients who met the WHO classification between February 1995 and June 2006.2 We excluded (1) 6 patients under 15 years of age, (2) 16 with primary cutaneous ALCL, (3) 1 with secondary systemic ALCL from Hodgkin's disease, (4) 2 who died before receiving any induction therapy including chemotherapy, radiation, and resection, (5) 4 whose medical records were insufficient to determine their clinical features and outcomes. Thus, our patient cohort consisted of 36 patients with adult primary systemic ALCL.
The medical records of these 36 adult patients with primary systemic ALCL were retrospectively reviewed. Diagnoses were based on a combination of routine histology, immunohistochemistry, cytogenetics, molecular genetics, and/or flow cytometry. The morphology was reviewed in all 36 cases. The available cases were tested for CD3, CD4, CD8, CD20, CD30, CD79a, ALK-1, UCHL-1, and PAX-5 to exclude B-cell lineage. Immunohistochemical analysis of ALK expression was performed on performed on 4 µm-thick paraffin sections taken from paraffin blocks of tissue samples from 30 patients. The biopsies of the remaining 6 patients were processed at outside institutions or obtained too long ago, making their paraffin blocks unavailable. Using the standard avidin-biotin-peroxidase technique, immunostaining with mouse monoclonal antibody to ALK (1 : 25; DAKO Diagnostics, Glousrup, Denmark) was performed in the automated immunostainer after heat-induced antigen retrieval protocols (Ventana ES automated immunohistochemistry stainer, Tucson, AZ, USA). Hyperplastic tonsils and a known case of ALK+ ALCL were used as negative and positive controls, respectively.
The clinical features evaluated for potential prognostic importance were gender, age, Ann Arbor stage, B symptoms, performance status, type and number of extranodal sites, and serum lactate dehydrogenase (LDH) level. Performance status was assessed according to the Eastern Cooperative Oncology Group (ECOG) scale.
Thirty-five patients (97%) received induction chemotherapy. One patient did not receive any chemotherapy and radiotherapy after complete surgical excision of localized cervical lymphadenopathy and had been in continuous CR for 17 months. Of 35 patients treated with chemotherapy, 31 (89%) received CHOP regimen; 1 received CHOP regimen and cytarabine together; 1 received regimens that included cyclophosphamide, etoposide, vincristine, bleomycin, methotrexate, and prednisolone plus or minus cytarabine (Vanderbilt);11 1 patient received vincristine, methotrexate, dexamethasone, ifosfamide, etoposide, cytarabine; vincristine, methotrexate, dexamethasone, cyclophosphamide, and doxorubicin in addition to intrathecal injection of methotrexate, cytarabine, and hydrocortisone (BNHL); and 1 received other regimens. Response was assessed according to IWC criteria.12
Overall survival (OS) was measured from diagnosis to death from any cause, with surviving patient follow up censored at the last contact date. EFS was defined as the time from the first therapy to the first occurrence of relapse, progression, or death from any cause. Follow up of patients not experiencing any of these events was censored at their date of last contact. To compare the clinical characteristics between ALK+ and ALK ALCL, independent t test was used for age and Chi-square test was used for the other factors. OS and EFS distributions were calculated using Kaplan and Meier methods. Survival curves were compared by log-rank or Breslow test according to the presence of cross line on the survival curve. Tests for comparison were regarded as significant if the p value was less than 0.05.
The demographic and clinical characteristics of the 36 patients are shown in Table 1. Of the 36 patients, 29 (81%) were male. The median age was 39 years (range, 17 - 67 years). Five patients (14%) were classified as Ann Arbor stage I, 5 (14%) as stage II, 11 (30%) as stage III, and 15 (42%) as stage IV. Lymphadenopathy was present in 30 patients (83%) at diagnosis: intraabdominal and retroperitoneal lymphadenopathy in 19 (63%), and mediastinal lymphadenopathy in 11 (37%). Splenomegaly was observed in 8 patients (22%). Twenty-six patients (72%) presented with extranodal involvement and 15 (42%) showed involvement at 2 or more extranodal sites. Six patients (17%) were assessed as having primary extranodal lymphoma without any nodal involvement. Bone (n = 7, 19%) and soft tissue (n = 6, 17%) were the most frequently involved sites, followed by the lung, pleura, muscle, bone marrow, and liver (n = 5, each). Soft tissue sites included mesenteric, peritoneal, and retroperitoneal soft tissue masses. Involvement of the gastrointestinal system including the stomach (n = 2) and small bowel (n = 3) was observed in 5 patients (14%). Thirty-two patients (89%) were scored as ECOG scale 1 or 2 and 4 (11%) as ECOG scale 3 or 4. B symptoms occurred in 21 patients (58%), and serum LDH was elevated in 25 (70%). Twenty-two patients (61%) were classified as low and low-intermediate IPI score and 14 (39%) as high-intermediate and high IPI score. However, 24 patients (66%) were scored as high-intermediate and high age-adjusted IPI.
ALK staining was performed again in 30 patients (83%) by a pathologist because the biopsy specimens from 6 patients were unavailable. We defined the cases, showing any nuclear or cytoplasmic positivity, as ALK+ ALCL. Of these 30 patients, 13 (43%) were ALK+ and 17 (57%) were ALK (Table 2). The median age of ALK+ patients (33 years; range, 19 - 49 years) was significantly lower than that of ALK patients (43 years; range, 17 - 67 years) (p = 0.047) but male to female ratio (5.5 vs. 3.3, p = 0.469), percent of patients with advanced stage (III, IV) disease (85 vs. 59%, p = 0.130), and percent of patients with extranodal involvement (77 vs. 76%) did not differ significantly. Eight ALK+ patients (62%) and 11 ALK- (65%) were low and low-intermediate IPI (p =0.861), and 4 and 7, respectively, were low and low-intermediate age-adjusted IPI (p = 0.421). As a whole, 8 survived and 5 died out of 13 ALK+ patients and 8 survived and 9 died out of 17 ALK patients.
Thirty-five patients (97%) were treated with combination chemotherapy regimens. The overall response rate was 91% with CR in 51% (n = 18) and PR in 40% (n = 14). Thirty-two patients (89%) received CHOP-based chemotherapy regimen. The overall response rate of CHOP-based chemotherapy regimen was 91% with CR in 50% (n = 16) and PR in 41% (n = 13). Two progressive disease (PD) and 1 unknown response were noted. Eighteen of 35 patients (50%) who received chemotherapy relapsed; 15 patients within 12 months of diagnosis, with the others showing relapse after 32, 40, and 52 months, respectively. In 7 patients, the relapse sites included previous disease sites whereas in 5, recurrences were at both previous and new sites and 4 relapsed only at new sites. Relapse sites of the remaining 2 patients were not reported.
Seventeen of 18 relapsed patients received second-line chemotherapy: 4 received cytarabine, cisplatin, and dexamethasone (DHAP); 3 received Vanderbilt;11 3 received etoposide, methylprednisolone, cytarabine, and cisplatin (ESHAP); and 7 received other regimens including CDDP plus taxol and T-LBL. It showed 2 CRs (12%); 3, PRs (18%); 1, stable disease (SD) (6%); and 8, PDs (47%), respectively, and the medical records of 3 patients were insufficient to assess the clinical responses of second-line chemotherapy.
One patient who relapsed after first-line chemotherapy and 2 after second-line chemotherapy were treated with high-dose chemotherapy, followed by autologous peripheral blood stem cell transplantation. One patient has remained in continuous CR for 8 years and 1 had a 23-month response whereas the third had a transient response with 2-month duration from relapse to death.
The median follow-up period was 47 months. OS rate was 57% at 2 years and 45% at 5 years, while EFS rate was 45% at 2 years and 15% at 5 years (Fig. 1). Median OS and EFS were 49 and 17 months, respectively.
Univariate analysis showed that performance (p = 0.035), IPI (p = 0.025), and age-adjusted IPI (p = 0.034) were significantly related to OS (Table 3 and Fig. 2). The survival rates were 61.5 and 20.0% in ALK+ and ALK ALCL groups, respectively. However, OS did not differ significantly between ALK+ and ALK groups (p = 0.483) (Fig. 3).
Sixteen patients (44%) died during the study period, 8 from progression of ALCL including superior vena cava syndrome, venoocclusive disease proven by autopsy and acute respiratory distress syndrome (ARDS) due to lung metastasis. Six patients died of infection and 2 died of unknown causes. Among the 6 patients who died of infection, 4 were diagnosed as sepsis or septic shock with neutropenia, 1, biliary sepsis due to hepatic infiltration of lymphoma, and the other, ARDS resulting from pneumonia. The parents or responsible individuals of some patients agreed to DNR (Do Not Resuscitate), therefore, some patients were discharged to the primary hospital with no hope of recovery.
Our retrospective analysis of 11 years' experience at a single institution contributes to our deeper understanding of the clinical features, responses to treatment, survival, and prognosis in adult patients with primary systemic ALCL in Korea.
We found that the clinical features of adult patients with primary systemic ALCL in Korea were similar to those of Western countries in that ALCL affects mostly young men and frequently presents with advanced disease. The male/female ratio (4.1), however, was markedly higher than those found in Western studies (1.4 - 1.8).1,13 On the other hand, the ratio was similar to those shown at other Korean and Asian institutions.14,15 Genetic background might be attributed to this difference, nevertheless, it should be confirmed by further prospective multi-center studies with molecular genetic analysis in a larger number of patients.
The main epidemiologic feature was a relatively higher incidence in the fourth (28%) and fifth (28%) decades of life and a median age of 39 years, a finding in close agreement with previous reports,16,17 but in disagreement with other earlier studies that showed a bimodal age distribution with a larger peak in the second and third decades and a smaller peak in the sixth and seventh decades.9 However, the distribution of elderly patients could be underestimated because elderly patients are generally reluctant to receive chemotherapy in Korea. For example, 3 patients over 60 years of age were excluded during enrollment in this study. One patient who died before receiving any induction therapy was 62 years old, and 2 whose medical records were insufficient were 66 and 77 years old, respectively. They refused to receive any chemotherapy.
In many Western studies, ALK expression has been shown to correlate with young age, systemic disease, and good prognosis.18-21 In our study period, immunohistochemical staining of ALK protein was repeated and confirmed prognostic importance in most available cases (30/36). We found that ALK patients tended to be older than ALK+ patients, however, these groups did not differ in extranodal involvement, advanced disease, high risk IPI and age-adjusted IPI group assignments. In addition, we found that these groups showed no significant differences in OS and EFS rate, findings similar to those previously reported.17,22 The differences observed between Korean and Western patients in small descriptive studies should be handled with care.
Five out of 13 patients with ALK+ ALCL died within 1 year of diagnosis. Their stages were all classified as III or IV. Extranodal involvements were observed in the lung, liver, soft tissue, or bone marrow. Bone marrow involvement might have provoked pancytopenia and made patients vulnerable to sepsis. Biliary sepsis was caused by hepatic infiltration of lymphoma cells. These characteristics of the 5 patients who died within 1 year of diagnosis affected the cross line of the 2 survival courses in spite of actual good prognosis in the AKL+ group.
Primary systemic ALCL often takes an aggressive clinical course for which combination therapy is warranted. It is not easy to directly compare response rate to each treatment regimen because various chemotherapy regimens were applied in previous studies, and primary cutaneous ALCL patients who had better prognosis were included in some studies.1,13,16,23 In Korea, our CR rate (51%) of whole chemotherapy regimen was lower than that (62%) found in previous study that enrolled only primary systemic ALCL patients.14 Meanwhile, the proportion of CHOP-based regimen reached up to 91% (32/35) of our whole chemotherapy regimens. The overall response rate of CHOP-based regimen was 91% with CR in 50% and PR in 41%. Therefore, our protocol is considerably homogenous to compare with previous reports, showing 50%.14 Moreover, high dose chemotherapy followed by autologous peripheral blood stem cell transplantation might be acceptable in relapsed patients but the small number of cases makes it difficult to draw a firm conclusion.
The data, which were derived from a small number of patients enrolled and at a single tertiary center, might cause a selection bias in this study. The small number of enrolled patients may be due to low incidence of adult primary ALCL in Korea. According to the 2002 Annual Report of the Korea Central Cancer Registry, only 1.9% of non-Hodgkin's lymphoma patients have ALCL. Another limitation was that we could not include analysis of recently reported common prognostic factors of primary ALCL (CD56, MUC-1, and survivin) on 30 patients available. It might be a good topic for future studies on ALCL.
In summary, we found that advanced disease and extranodal involvement are frequent, and confirmed male predominance in Korean adult primary systemic ALCL patients. OS was 49 months, and CR and PR rates to CHOP-based chemotherapy were 50 and 41%, respectively. Univariate analysis showed that performance status, IPI, and age-adjusted IPI score predicted OS, but not ALK expression. For further evaluation of optimal treatment of Korean ALCL patients, a large national or multi-institutional prospective study is needed.
Figures and Tables
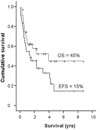 | Fig. 1OS and EFS of 36 patients with primary systemic ALCL (median 49.0 and 17.0 months; 5-years survival rate 45 and 15%, respectively). OS, overall survival; EFS, event-free survival. |
 | Fig. 2OS according to performance and International Prognostic Index in 36 patients. OS, overall survival; IPI, International Prognostic Index. |
 | Fig. 3OS and EFS of 30 patients with ALK+ and ALK- primary systemic ALCL (median 17.0 months; 2-year EFS rate 45%; 5-year EFS rate 15%). OS, overall survival; EFS, event-free survival; ALK, anaplastic lymphoma kinase; ALCL, anaplastic large cell lymphoma. |
Table 2
Comparison of 30 Patients with ALK+ and ALK-- Primary Systemic Anaplastic Large Cell Lymphoma

ECOG, the Eastern Cooperative Oncology Group; LDH, lactate dehydrogenase; IPI, International Prognostic Index.
Chi-square test was used to compare with other factors except for age.
*Independent t test was used to compare with other factors except for age.
†Primary extranodal lymphoma without any lymph node involvement.
References
1. Tilly H, Gaulard P, Lepage E, Dumontet C, Diebold J, Plantier I, et al. Primary anaplastic large-cell lymphoma in adults: clinical presentation, immunophenotype, and outcome. Blood. 1997. 90:3727–3734.

2. Chan JK. The new World Health Organization classification of lymphomas: the past, the present and the future. Hematol Oncol. 2001. 19:129–150.

3. Stein H, Mason DY, Gerdes J, O'Connor N, Wainscoat J, Pallesen G, et al. The expression of the Hodgkin's disease associated antigen Ki-1 in reactive and neoplastic lymphoid tissues: evidence that Reed-Sternberg cells and histiocytic malignancies are derived from activated lymphoid cells. Blood. 1985. 66:848–858.

4. Falini B, Pileri S, Pizzolo G, Dürkop H, Flenghi L, Stirpe F, et al. CD30 (Ki-1) molecule: a new cytokine receptor of the tumor necrosis factor receptor superfamily as a tool for diagnosis and immunotherapy. Blood. 1995. 85:1–14.

5. Harris NL, Jaffe ES, Stein H, Banks PM, Chan JK, Cleary ML, et al. A revised European-American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood. 1994. 84:1361–1392.

6. Stein H, Foss HD, Dürkop H, Marafioti T, Delsol G, Pulford K, et al. CD30(+) anaplastic large cell lymphoma: a review of its histopathologic, genetic, and clinical features. Blood. 2000. 96:3681–3695.
7. Mason DY, Bastard C, Rimokh R, Dastugue N, Huret JL, Kristoffersson U, et al. CD30-positive large cell lymphomas ('Ki-1 lymphoma') are associated with a chromosomal translocation involving 5q35. Br J Haematol. 1990. 74:161–168.

8. Duyster J, Bai RY, Morris SW. Translocations involving anaplastic lymphoma kinase (ALK). Oncogene. 2001. 20:5623–5637.

9. Skinnider BF, Connors JM, Sutcliffe SB, Gascoyne RD. Anaplastic large cell lymphoma: a clinicopathologic analysis. Hematol Oncol. 1999. 17:137–148.

10. Campo E, Chott A, Kinney MC, Leoncini L, Meijer CJ, Papadimitriou CS, et al. Update on extranodal lymphomas. Conclusions of the Workshop held by the EAHP and the SH in Thessaloniki, Greece. Histopathology. 2006. 48:481–504.

11. Waits TM, Greco FA, Greer JP, Johnson DH, Wolff SN, Stein RS, et al. Effective therapy for poor-prognosis non-Hodgkin's lymphoma with 8 weeks of high-dose-intensity combination chemotherapy. J Clin Oncol. 1993. 11:943–949.

12. Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM, et al. Report of an international workshop to standardize response criteria for non-Hodgkin's lymphomas. NCI Sponsored International Working Group. J Clin Oncol. 1999. 17:1244–1253.
13. Longo G, Fiorani C, Sacchi S, Callea V, Lombardo M, Federico M, et al. Clinical characteristics, treatment outcome and survival of 36 adult patients with primary anaplastic large cell lymphoma. Gruppo Italiano per lo Stufio dei Linfomi (GISL). Haematologica. 1999. 84:425–430.
14. Park SR, Baek JY, Kim DW, Im SA, Kim TY, Bang YJ, et al. Primary systemic anaplastic large cell lymphoma in a single Korean institution: clinical characteristics and treatment outcome. J Korean Med Sci. 2006. 21:633–638.
15. Lin CN, Hou CC, Hwang WS, Chuang SS. Anaplastic large cell lymphoma--a rare disorder in Southern Taiwan. Leuk Lymphoma. 2003. 44:1727–1731.

16. Clavio M, Rossi E, Truini M, Carrara P, Ravetti JL, Spriano M, et al. Anaplastic large cell lymphoma: a clinicopathologic study of 53 patients. Leuk Lymphoma. 1996. 22:319–327.

17. Au WY, Ma SY, Chim CS, Choy C, Loong F, Lie AK, et al. Clinicopathologic features and treatment outcome of mature T-cell and natural killer-cell lymphomas diagnosed according to the World Health Organization classification scheme: a single center experience of 10 years. Ann Oncol. 2005. 16:206–214.

18. ten Berge RL, Oudejans JJ, Ossenkoppele GJ, Meijer CJ. ALK-negative systemic anaplastic large cell lymphoma: differential diagnostic and prognostic aspects--a review. J Pathol. 2003. 200:4–15.

19. Falini B, Pileri S, Zinzani PL, Carbone A, Zagonel V, Wolf-Peeters C, et al. ALK+ lymphoma: clinico-pathological findings and outcome. Blood. 1999. 93:2697–2706.
20. ten Berge RL, Oudejans JJ, Ossenkoppele GJ, Pulford K, Willemze R, Falini B, et al. ALK expression in extranodal anaplastic large cell lymphoma favours systemic disease with (primary) nodal involvement and a good prognosis and occurs before dissemination. J Clin Pathol. 2000. 53:445–450.

21. Gascoyne RD, Aoun P, Wu D, Chhanabhai M, Skinnider BF, Greiner TC, et al. Prognostic significance of anaplastic lymphoma kinase (ALK) protein expression in adults with anaplastic large cell lymphoma. Blood. 1999. 93:3913–3921.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download