Abstract
Angioma serpiginosum is an uncommon, acquired vascular nevoid disorder with capillary dilation and proliferation in the papillary dermis. The eruptions are asymptomatic and characterized by grouped, erythematous to violaceous, serpiginous and punctate macules. The condition usually appears in females during adolescence on unilateral lower extremities and the buttocks. We report a rare case with a late onset and atypical distribution of lesions in a 48-year-old female patient who had groups of punctate lesions on her left foot for four to five years. Histopathological examination showed hyperkeratosis and multiple dilated and proliferated capillaries in the papillary dermis. Inflammation and extravasation of red blood cells were not found. According to the clinical and pathological findings, we established a diagnosis of angioma serpiginosum. She was treated with a pulsed dye laser, and the angiomatous lesions subsequently improved.
Angioma serpiginosum is a rare acquired vascular nevoid disorder that is histopathologically characterized by the dilation and proliferation of superficial capillaries in the dermis. The condition is most common in females, often prominent on the lower legs and buttocks, usually apparent in late childhood, and slowly progressive.1 Angioma serpiginosum is clinically characterized by asymptomatic, serpiginous, grouped, red and punctate macules often on a bed of erythema.2 Although involution may occur, it is never complete. Treatment with a pulsed dye laser may improve or eliminate the disorder.3 We report a rare case of angioma serpiginosum originating on the left foot in a 48-year-old female patient who was treated with a pulsed dye laser.
A 48-year-old female patient presented with a history of slowly progressive asymptomatic, purpuric to erythematous eruptions on her left foot for 4 to 5 years. These skin lesions had initially been inspected by an internist for investigation of possible vasculitis or purpura. The patient had no significant past medical history of diabetes, hypertension or drug or trauma history prior to the onset of her lesions. No member of her family had similar lesions. She had received hormone replacement therapy for 3 years that was discontinued 3 months prior to the examination. She felt that the lesions progressed rapidly during the 3 year period. Laboratory data, including complete blood count, platelet aggregation tests, prothrombin time, partial thromboplastin time, bleeding time, erythrocyte sedimentation rate, estrogen, progesterone, and other biochemistries, were within normal limits.
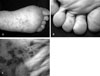
She was referred to a dermatologist, and the examination revealed multiple grouped, nonblanchable, red to violaceous macules on an erythematous background extending from the left sole to the toes (Figs. 1A, B, and C). On close examination with a hand lens, the blood contents of the lesions were clearly within capillary loops without evidence of hemosiderin deposition. A skin biopsy specimen revealed hyperkeratosis and many dilated and proliferated capillaries in the papillary dermis. No significant inflammatory cell infiltration or red blood cell extravasation was seen (Figs. 2A and B). Estrogen and progesterone receptors were negative. Correlation of the clinical and histological findings resulted in the diagnosis of angioma serpiginosum.
Subsequently, the patient was treated with a pulsed dye laser at 585 nm wavelength, 450 µs pulse duration and 5 mm spot diameter, using contiguous non-overlapping spots. The patient received four sessions using an energy density of 9 - 12 J/cm2 at 4-week intervals with a good response.
Angioma serpiginosum, an unusual vascular tumor, was first described by Hutchinson in 1889 as a peculiar form of serpiginous and "infective" nevoid disease and was named by Crocker in 1894.4 The disorder is characterized by minute, punctate, purple-colored to bright-red macules that have a tendency to become papules. The papules occur in groups, which enlarge through the formation of new lesions at the periphery, while those at the center fade. In this manner, small rings or serpiginous patterns are formed.3 Lesions are asymptomatic and do not disappear under diascopy. Characteristic findings of angioma 'red lagoons' by epiluminescence microscopy can distinguish angioma serpiginosum from purpuric dermatoses.5
Although angioma serpiginosum affects both genders at all ages, 90 percent of the cases occur in girls and 80 percent of cases originate before the age of 20.6 The condition is usually sporadic, although familial cases with an autosomal dominant inheritance pattern have also been reported.7 The lesions may occur anywhere, but the predominant sites are the lower extremities. Although the palms, soles, and mucous membranes are typically spared,4 angioma serpiginosum appearing on the sole have been reported.6 Most cases are unilateral, but rare bilateral cases with extensive cutaneous involvement are also seen.8 In addition, angioma serpiginosum has also been reported in association with retinal and spinal angioma.9,10 Our patient had no associated manifestations. The unusual features in our case were the late onset, and the distribution of the lesions on the sole, toes, and lateral side of the left foot. To the best of our knowledge, this unusual presentation of angioma serpiginosum has never been reported.
A histopathologic examination of the skin biopsies of our patient showed the characteristic features of angioma serpiginosum, with increased numbers of dilated and proliferated capillaries in the upper dermis without the significant presence of inflammatory cell infiltration, red blood cell extravasation, and hemosiderin.4 Some authors consider angioma serpiginosum as a neoplasm because of endothelial cell proliferation with formation of new capillaries.11 Other authors consider the condition a malformation because of the abnormal morphogenesis in the form of thickened capillary walls secondary to precipitation of fibrillar material admixed with a few collagen fibers. In addition, there is the formation of an accessory lumen or slit-like protrusion of the lumen into the endothelial lining.12
The pathogenesis of angioma serpiginosum is unknown. Increased levels of estrogen may play a role in the development of angioma serpiginosum because most cases arise in girls under 16 years old and progress rapidly during pregnancy.1,10 This progression may be attributed to the ability of estrogen to enhance the proliferation of hemangioma vascular endothelial cells.13 In our case, the lesions progressed quickly after the patient started hormone replacement therapy, and slowed down after stopping hormone replacement therapy. This finding may indicate estrogen dependence. We hypothesized that hypersensitivity of the estrogen and progesterone receptors located on the surface of the endothelial cells in the skin lesions or increased blood levels of estrogen and progesterone contributed to the disease progression. However, the patient's blood levels of estrogen and progesterone were normal, and the estrogen and progesterone receptors were negative in the lesions. The levels of estrogen and progesterone may be elevated during hormone replacement therapy. In addition, Neumann suggested that the lesions may simply represent an abnormal vascular response to cold. Dermal vessel damage due to cold exposure, possibly in association with other yet unknown factors, may cause the formation of new capillaries that subsequently aggregate to form larger ecstatic vessels.14 Our patient did not recall any exposure to severe cold temperatures.
Angioma serpiginosum must be differentiated from the unilateral nevoid telangiectasia syndrome, angiokeratoma, acquired port-wine nevus, and pigmented purpuric dermatoses. All these diseases have dilated capillaries in the dermis, except for pigmented purpuric dermatoses. Angiokeratoma are pathologically characterized by the presence of papillomatosis and hyperkeratosis. Pigmented purpuric dermatoses show hemosiderin deposition, extravasated red cells, and inflammation. Unilateral nevoid telangiectasia syndrome is a rare congenital or acquired (estrogen-related, alcohol/hepatic-related) condition characterized by a dermatomal distribution of telangiectasia, over the unilateral C3-T1 dermatomes.15 Acquired port-wine nevus is generally found unilaterally on the face and neck with nearly half of all facial port-wine nevus localized in the distribution of the trigeminal nerve. The main histopathological characteristic of acquired port-wine nevus are multiple dilatations and ectasias of capillaries in the middle to superficial dermis, with a smaller degree of proliferation than that seen with angioma serpiginosum. In clinical practice, there is one significant characteristic that can easily distinguish the lesions of angioma serpiginosum from chronic purpura. Upon closer inspection with a lens, the distinguishing characteristic is that angioma serpiginosum shows intravascular lesions that are composed of tiny spots and streaks with pallid domains between them, whereas chronic purpura reveals extravascular blood.1 Clinical history and physical examination may supply the major distinction between these two conditions to establish an appropriate diagnosis without further work-up.
Partial or complete spontaneous regression of the lesions may occur, but usually the condition is slowly progressive during the first few years. The lesions are usually responsive to argon or dye lasers. Landthaler and coworkers treated two patients with angioma serpiginosum using an argon laser with good results.16 Polla and colleagues treated three patients with angioma serpiginosum using a tunable pulsed dye laser.17 In two patients, there was more than 75% clearance, and in one patient, the clearance was between 25 and 50%. Long and coworkers treated five patients with angioma serpiginosum using a pulsed tunable dye laser at 585 nm, 450 µs pulse duration and 5 mm spot diameter.18 All patients received 2 to 8 sessions using energies of 6.5 - 7.25 J/cm2 at 8-week intervals. The lesion resolved in one patient, virtually resolved in two, and excellent improvement was seen in one. In our case, the patient was treated with a pulsed dye laser at 585 nm, 450 µs pulse duration, and 5 mm spot diameter, using contiguous non-overlapping spots. She received four sessions using an energy density of 9 - 12 J/cm2 at 4-week intervals with a good response. However, the patient was lost to follow-up, and we could not do further treatment or evaluation of the condition. The 585 nm pulsed dye laser at pulse duration of 450 µs produces a thermal injury effectively confined to blood vessels as the laser pulse duration is shorter than the thermal relaxation time for cutaneous blood vessels.19 This results in a limited depth of vascular injury of up to 1 - 2 mm and is effective in treating superficial vascular disorders, such as telangiectases, superficial capillary hemangioma, port-wine stain, and spider nevi.20 The superficial nature of angioma serpiginosum is consistent with the good results obtained by pulsed dye laser therapy.18
Our patient is unusual because of the late onset age, rare distribution, and the possible association with hormone replacement therapy. We hope that our report of this case in a patient with a disguiser of purpura will facilitate correct diagnosis in other patients. The results also suggest that the treatment with a pulsed dye laser is effective for angioma serpiginosum.
Figures and Tables
References
1. Cox NH, Paterson WD. Angioma serpiginosum: a simulator of purpura. Postgrad Med J. 1991. 67:1065–1066.

2. Al Hawsawi K, Al Aboud K, Al Aboud D, Al Githami A. Linear angioma serpiginosum. Pediatr Dermatol. 2003. 20:167–168.

4. Hunt SJ, Santa Cruz DJ. Acquired benign and "borderline" vascular lesions. Dermatol Clin. 1992. 10:97–115.
5. Ohnishi T, Nagayama T, Morita T, Miyazaki T, Okada H, Ohara K, et al. Angioma serpiginosum: a report of 2 cases identified using epiluminescence microscopy. Arch Dermatol. 1999. 135:1366–1368.
7. Marriott PJ, Munro DD, Ryan T. Angioma serpiginosum-familial incidence. Br J Dermatol. 1975. 93:701–706.

8. Katta R, Wagner A. Angioma serpiginosum with extensive cutaneous involvement. J Am Acad Dermatol. 2000. 42:384–385.

9. Gautier-Smith PC, Sanders MD, Sanderson KV. Ocular and nervous system involvement in angioma serpiginosum. Br J Ophthalmol. 1971. 55:433–443.

10. Erbagci Z, Erbagci I, Erkiliç S, Bekir N. Angioma serpiginosum with retinal involvement in a male: a possible aetiological role of continuous cold exposure. J Eur Acad Dermatol Venereol. 2004. 18:238–239.

11. Requena L, Sangueza OP. Cutaneous vascular proliferation. Part II. Hyperplasias and benign neoplasms. J Am Acad Dermatol. 1997. 37:887–919. quiz 920-2.
13. Xiao X, Hong L, Sheng M. Promoting effect of estrogen on the proliferation of hemangioma vascular endothelial cells in vitro. J Pediatr Surg. 1999. 34:1603–1605.

14. Neumann E. Some new observations on the genesis of angioma serpiginosum. Acta Derm Venereol. 1971. 51:194–198.
15. Tok J, Berberian BJ, Sulica VI. Unilateral nevoid telangiectasia syndrome. Cutis. 1994. 53:53–54.
16. Landthaler M, Haina D, Waidelich W, Braun-Falco O. A three-year experience with the argon laser in dermatotherapy. J Dermatol Surg Oncol. 1984. 10:456–461.
17. Polla LL, Tan OT, Garden JM, Parrish JA. Tunable pulsed dye laser for the treatment of benign cutaneous vascular ectasia. Dermatologica. 1987. 174:11–17.
18. Long CC, Lanigan SW. Treatment of angioma serpiginosum using a pulsed tunable dye laser. Br J Dermatol. 1997. 136:631–632.

19. Wheeland RG. Treatment of port-wine stains for the 1990s. J Dermatol Surg Oncol. 1993. 19:348–356.
20. Spicer MS, Goldberg DJ. Lasers in dermatology. J Am Acad Dermatol. 1996. 34:1–25. quiz 26-8.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download