Abstract
Purpose
c-fos expression in spinal neurons that are activated by lower urinary tract stimulation are not organ specific. In this experiment, we demonstrated changes of c-fos expression in bladder-specific preganglionic neurons (PGNs) and interneurons using pseudorabies virus (PRV).
Materials and Methods
Forty Sprague-Dawley rats were used. We identified the neuronal pathway associated with the bladder by injecting PRV into the detrusor. An immunohistochemical method was used to stain Fos-protein encoded by the c-fos gene. Immunofluorescent staining for PRV was performed to evaluate changes in bladder-specific spinal neurons.
Results
Immunofluorescent staining with choline acetyltransferase (ChAT) revealed that the sacral parasympathetic nucleus (SPN) regions contained 9.8 PGNs/section. In rats with chronic spinal cord injury by intravesical saline instillation, 82.4 ± 10.3% of PGNs in SPN exhibited Fos-immunoreactive (IR). Two and a half days after PRV infection, PRV-IR PGNs were observed at 5.4 PGNs/ section, and 2.7 ± 1.6% of them exhibited Fos-IR. Unlike ChAT-IR PGNs, PRV-IR PGNs are bladder-specific neurons and PRV-IR and Fos-IR cells found in the back of PRV-IR PGNs are bladder-specific interneurons. Three days after PRV infection, we observed many PRV-IR and Fos-IR cells in the dorsal commissure. These neurons are interneurons distributed in the bladder.
Conclusion
We confirmed that in chronic spinal cord injury, the patterns of c-fos expression in bladder-specific spinal neurons were similar to those in voiding-reflex related spinal neurons, which had already been demonstrated earlier. We believe that our methodology can be applied to study interactions between voiding and other organs as well, such as the urethra and prostate.
Voluntary micturition of the lower urinary tract is regulated by a complex mechanism in the spinal and supraspinal neural pathways. Spinal cord injury rostral to the lumbosacral level alters the coordination between the bladder and external urethral sphincter and chronically impairs voluntary micturition. The changes that occur in spinal voiding reflexes after spinal cord injury (SCI) appear to be of a similar nature in humans as well as in experimental animals, and they started to provide important insight into a variety of neurogenic disorders of the lower urinary tract (LUT). Recent studies1-4 have demonstrated that chronic SCI in rats can result in changes in the neurochemical properties of bladder afferent and spinal cord pathways. In spinalized rats, the properties of C-fiber afferents are altered so that they increase their excitability to induce bladder hyperreflexia.5-7 These changes suggest substantial reorganization of reflex connections in the spinal cord and marked changes in the properties of micturition reflex pathways of post-chronic SCI.
c-fos is a proto-oncogene that encodes Fos-protein in the central nervous system.8 It is known as an indicator for postsynaptic activation of spinal cord neurons that receive afferent input from the LUT, including the bladder, urethra, and perineum. Previous experiments3,4,8,9 have used immediate early c-fos expressions to identify neurons in the spinal cord that receive afferent input from the LUT and revealed that bladder distension or chemical irritation of the LUT of rats produced increased number and altered distribution pattern of Fos-IR cells in discrete regions of the L6-S1 spinal cord, including the superficial lateral and medial dorsal horn (LDH, MDH, respectively), dorsal commissure (DCM), and SPN regions. Fos-IR neurons contain several cell types including PGNs, interneurons, and spinal tract neurons projecting to the brainstem and diencephalons.10 However, those spinal neurons that are activated by LUT stimulation are not organ-specific neurons; that is, if we simply use c-fos, we cannot observe changes in spinal neurons that can be attributed, respectively, to different organs such as the bladder and urethra.
In this study, we proposed a method to address this problem. We exploited the fact that PRV can act as an organ-specific transneuronal tracer and used the spinalized rat model and activated it by using LUT afferent input. The primary purpose was to observe post-activation of the c-fos expression in bladder-specific PGNs and interneurons.
We used a total of 40 adult Sprague-Dawley female rats weighing 200 - 300g. The experiment was conducted in 2 parts.
We conducted this part of the experiments to confirm whether PRV injection into the spinal neuron affects c-fos expression.
Thirty normal rats were divided into 4 groups: (i) sham (n = 4), (ii) sham with PRV (PRV detrusor injection; n = 10), (iii) sham with acetic acid (acetic acid bladder instillation; n = 6), and (iv) sham with PRV and acetic acid (acetic acid instillation applied 3 days after PRV injection; n = 10). For sham operation, we incised the lower abdomen and inserted a catheter into the bladder. For acetic acid instillation, we infused 1% acetic acid through a polyethylene catheter (PE-50) 2h prior to sacrificing the rats.
We used a total of 10 spinal cord-injured rats. We injected PRV into the bladder and collected specimens after killing the rats at different intervals (1.5, 2.0, 2.5, 3.0 days after PRV injection). As with normal rats, we conducted saline instillation 2h prior to sacrificing the rats.
We began by subcutaneously injecting acerpromazine (0.05 mg/kg), ketamine (50 mg/kg), and xylazine (5 mg/kg) and using enflurane as general anesthesia. We then incised the back skin, removed the T9-T10 vertebrae, and transected the T9 spinal cord. After filling the space between the ends of transected spinal cord with Gelfoam (Johnson and Johnson Medical Limited, Skipton, UK), the incised site was sutured. We injected prophylactic antibiotics (ampicillin 150 mg/kg) 1 day before the procedure. Following the procedure, we periodically injected antibiotics for 1 week. We maintained a healthy condition for these specimens and assisted their urination by pressing their bladder twice a day until they could get out of spinal shock.
The Bartha strain of PRV (timely donated by Bong Hee Lee, Cheju National University, Cheju, Korea) was used in this study. It is an attenuated strain of PRV that reliably label neuronal pathways via transneuronal transport. The titer of the virus stock determined by using PK15 cell line was 1 × 108 plaque forming units (pfu)/mL. After anesthetizing both normal rats and rats whose spinal cords had been injured 5 weeks earlier, we incised their lower abdomen and exposed the bladder. Afterwards, we injected 2 µL of PRV into both sides of the bladder using a 5 µL Hamilton syringe. We kept the rats under observation for varying periods (1.5 - 3.0 days).
Normal rats and spinalized rats were killed by intracardiac perfusion of 0.1 M phosphate buffer (PB; pH 7.4) followed by 4% paraformaldehyde fixative in PB (0.1 M, pH 7.4). The spinal cord sections (L6-S1) were removed and post-fixed for 12 h in the same fixative at 4℃ before being cryoprotected in 0.1 M phosphate-buffered 30% sucrose solution (pH 7.4) overnight. They were then sectioned (40 µm) on a freezing microtome (Microm, Walldorf, Germany).
Using the avidin-biotin complex (ABC) method, alternate sections (40 µm) of the spinal cord were processed for immunoreactivity to c-fos protein using nickel intensification.9 Sections were incubated first with rabbit anti-Fos (1 : 10000, Oncogene, Cambridge, MA, USA) for 24 h at 4℃, and in bionylated secondary antibody (1 : 1000, Chemicon) and ABC reagent (Chemicon), for 2 h each at room temperature. All sections were examined by bright field microscope.
For immunofluorescent staining, sections were exposed to a combination of rabbit anti-PRV (1 : 1000, Oncogene, Cambridge, MA, USA) and goat anti-Fos (1 : 1000, Cambridge, MA, USA) or goat anti-ChAT (1 : 1000, Chemicon, Temecula, CA, USA) and rabbit anti-Fos (1 : 5000, Oncogene, Cambridge, MA, USA). Sections were incubated at 4℃ for 24 h and treated with a combination of donkey antirabbit conjugated with FITC (1/50; Jackson Immunoresearch, West Grove, PA, USA) and donkey antigoat conjugated with TRIRC (1 : 50, Jackson Immunoresearch, West Grove, PA, USA). Sections were examined under epifluorescent illumination.
We determined the numbers of Fos-positive cells by counting positively stained neurons. The sections counted had to be separated by at least 100 µm to eliminate the possibility of double counting. We randomly selected 5 sections from the L6-S1 segment of normal rats, averaged Fos-positive cells in them, and calculated their mean and standard deviations.
Double immunofluorescent stainings, consisting of c-fos and PRV or c-fos and ChAT, were performed to evaluate changes in spinal neurons associated with detrusor muscle in spinalized rats. The figures for ChAT-IR and PRV-IR cells in the L6-S1 segment were calculated by counting the number of cells from each section and averaging them. The figures for Fos-IR cells are in percentages.
When we observed the samples 3 days after PRV injection, we detected no significant difference in the number of Fos-protein positive neurons between the sham and PRV groups. Likewise, we observed no significant difference in the number of Fos-positive neurons in the normal acetic acid instillation and the one preceded by PRV injection (Table 1). This indicates that PRV injection into the spinal neuron by itself neither stimulated nor suppressed c-fos expression.
Immunofluorescent staining with ChAT revealed that the SPN regions contained a number of ChAT-IR neurons (Fig. 1A). Consequently, they corresponded to PGNs. On average, 9.8 PGNs/section were stained with ChAT. Three days after virus infection, PRV-IR cells were discovered dorsal to the more ventrally located preganglionic neurons (PRV-IR neurons were commonly seen to lie just dorsal to the preganglionic ChAT-IR neurons in the SPN). Furthermore, PRV-IR cells tended to increase in the DCM (Fig. 1B).
In chronic SCI rats with intravesical saline instillation, many Fos-IR cells were found in the SPN region of the L6-S1 segment; 82.4 ± 10.3% of ChAT-IR PGNs in SPN exhibited Fos-IR. Cholinergic Fos-IR cells were located ventral to the cells exhibiting only Fos-IR (Fig. 2A).
Two and a half days after PRV infection, a number of PRV-IR cells were observed in SPN. The PRV-IR cells located in the ventral position of the SPN corresponded to PGNs. Unlike ChAT-IR PGNs, PRV-IR PGNs are neurons specifically innervated in the bladder. On average, PRV-IR PGNs were observed at 5.4 PGNs/section, and 2.7 ± 1.6% of them exhibited Fos-IR. Meanwhile, the PRV-IR cells---the Fos-IR cells found in the back of PRV-IR PGNs---are interneurons specifically distributed on the bladder. Spinal projection neurons were observed only as Fos-IR, and they were found in mixture with interneurons in the back of PGNs (Fig. 2B).
Three days after PRV infection, we observed many PRV-IR and Fos-IR cells in the DCM. These neurons were interneurons innervated in the bladder (Fig. 2C).
An important and crucial feature of PRV, compared to other methods of neuroanatomical tracing such as those based on fluorescent dyes or horseradish peroxidase (HRP), is that it is transmitted from infected neurons to other neurons via their synaptic connections. Therefore, it defines the specific pathways involved in neuron-to-neuron signaling.11,12 The virus does not travel from the periphery along bladder afferent pathways as readily as it does along the efferent pathways, or very few PRV-IR neurons are detected in the sacral spinal cord at 60 h in animals whose S1-S3 ventral roots have been transected prior to injection of the virus.13-15 Previous studies16,17 have shown that the PGN in the sacral spinal cord is the first infection site associated with PRV injection in the bladder. Infection then spreads to interneurons concentrated in an area dorsal to the ipsilateral PGN and a second area in the DCM. Therefore, PRV serves as a good tracer in studying organ-specific neurons. By injecting PRV into the detrusor, we set out to identify bladder-specific neurons among the voiding-related spinal neurons. However, it is important to remember that intermittent urination may also stimulate urethra and perineum when saline or acetic acid instillation in the bladder is conducted. Therefore, we cannot necessarily conclude for certain that changes of c-fos expression induced by LUT stimulation in spinal neurons are organ specific.
An experiment with PRV and c-fos has to be careful that PRV infection itself does not affect c-fos expression. Table 1 illustrates this point. It shows that the numbers of Fos-IR cells observed are not statistically significantly different among the sham operation group, PRV injection group, acetic acid instillation group, PRV injection, and acetic acid instillation group. In short, PRV infection in the spinal neuron by itself neither stimulates nor suppresses c-fos expression.
Vizzard3,4 studied the relationship between c-fos and PGNs by using ChAT in spinalized rat models and demonstrated that the positive neurons from the SPN of the L6 segment that become stained by ChAT can be thought of as PGNs while those neurons that are not susceptible to stain by ChAT can be thought of as inter neurons or projection neurons, thus observing 75.6% of the ChAT-IR PGNs as Fos-positive. In our experiment, the observed frequency of 82.4% was slightly higher. At any rate, both these results pertain only to voiding-related PGNs and do not represent bladder-specific PGNs. When PRV-IR PGNs were analyzed after injecting PRV into the bladder, we observed 5.4 PGNs/section. Comparing this figure to 9.8 PGNs/section for ChAT-IR PGNs, we concluded that these PGNs represent only a portion of the neurons found in the bladder. Additionally, only 2.7% of the PRV-IR PGNs were observed to be Fos-positive, again indicating that only a small fraction of the voiding-reflex-related PGNs are found in the bladder.
When we eliminate PGNs from PRV-IR and Fos-IR cells in the SPN, we are left with PRV-IR and Fos-IR interneurons, which appear to be bladder-specific interneurons. These PRV-IR interneurons were found in the back of PRV-IR PGNs and in mixture with projection neurons. Likewise, we again found bladder-specific neurons by using PRV and c-fos in the DCM.
Although the use of PRV is beneficial in allowing us to observe organ-specific neurons, it also makes it more difficult to ascertain exact quantification because the number of observed PRV-IR cells tends to vary with survival time. As of now, we may have to settle for observing only topographic distribution. A future project in this area is to quantify the PRV-IR cells.
The literature3,4,18 available in this area coarsely categorizes voiding-reflex related spinal neurons into PGNs, interneurons, and projection neurons, and focuses on their changes. By contrast, the significance of our experiment could be found in observing changes in bladder-specific neurons within different categories of neurons. Ultimately, we confirmed that the patterns of c-fos expression in bladder-specific spinal neurons in chronic SCI were similar to those in voiding-reflex related spinal neurons, which had already been demonstrated earlier. We believe that our present methodology can be applied to the study of interactions between voiding and other organs such as the urethra and prostate.
Figures and Tables
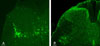 | Fig. 1Fluorescent photomicrographs of an L6 spinal cord section. Virus-labeled bladder neuron (green) at 3 days postinfection (A). ChAT-IR cells (green cytoplasm) in the SPN (B). Scale bar: 100 µm. |
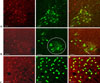 | Fig. 2Co-localization of c-fos, ChAT, and PRV in the L6-S1 segments. Double immunofluorescent staining showed that Fos-IR cells (red nuclei) were co-localized with ChAT-IR cells (green cytoplasm) in the region of the SPN (A). Fos (red) co-localized with PRV (green) after 2.5 days postinfection in the SPN. Based on the location of the cells, PRV-IR cells in the circle corresponded to PGNs. PRV-IR PGNs exhibited Fos-IR (arrow) (B). Merged immunofluorescence showed that Fos (red) was co-localized with PRV (green) in the DCM (C). Scale bar: 50 µm. |
Table 1
The Number of Fos-IR Cells/Section Measured in Each Groups

PRV, pseudorabies virus; AA, acetic acid; MDH, medial dorsal horn; LDH, lateral dorsal horn; DCM, dorsal commissure; SPN, sacral parasympathetic nucleus.
Sham operation only (n = 4), sham with PRV injection into bladder (n = 10), sham with acetic acid bladder instillation (n = 6), and sham with acetic acid instillation applied 3 days after PRV injection (n = 10).
Data are mean ± SE.
References
1. Kruse MN, Belton AL, de Groat WC. Changes in bladder and external urethral sphincter function after spinal cord injury in the rat. Am J Physiol. 1993. 264:R1157–R1163.

2. Vizzard MA. Alterations in growth-associated protein (GAP-43) expression in lower urinary tract pathways following chronic spinal cord injury (SCI). Somatosens Mot Res. 1999. 16:369–381.

3. Vizzard MA. Alternation in spinal cord Fos protein expression induced by bladder stimulation following cystitis. Am J Physiol. 2000. 278:1027–1039.
4. Vizzard MA. Increased expression of spinal cord Fos protein induced by bladder stimulation after spinal cord injury. Am J Physiol Regul Integr Comp Physiol. 2000. 279:R295–R305.

5. de Groat WC, Nadelhaft I, Milne RJ, Booth AM, Morgan C, Thor K. Organization of the sacral parasympathetic reflex pathways to the urinary bladder and large intestine. J Auton Nerv Syst. 1981. 3:135–160.

6. de Groat WC, Kawatani M, Hisamitsu T, Cheng CL, Ma CP, Thor K, et al. Mechanisms underlying the recovery of urinary bladder function following spinal cord injury. J Auton Nerv Syst. 1990. 30:S71–S78.

7. de Groat WC, Booth AM, Yoshimura N. Maggi CA, editor. Neurophysiology of micturition and its modification in animal models of human disease. Nervous control of the urogenital system: autonomic nervous system. 1993. London: Harwood Academic Publishers;227–290.
8. Birder LA, de Groat WC. Increased c-fos expression in spinal neurons after irritation of the lower urinary tract in the rat. J Neurosci. 1992. 12:4878–4889.

9. Birder LA, de Groat WC. Induction of c-fos expression in spinal neurons by nociceptive and nonnociceptive stimulation of LUT. Am J Physiol. 1993. 265:R326–R333.
10. Hamilton MO, Papka RE, O'donoghue DL, Vaidya AM, Williams SJ, Poff CR, et al. Spinal projection neurons to the laterodorsal pontine tegmental nucleus: relationship to preganglionic neurons and nitric oxide synthase. J Comp Neurol. 1995. 353:1–8.

11. Card JP, Rinaman L, Schwaber JS, Miselis RR, Whealy ME, Robbins AK, et al. Neurotropic properties of pseudorabies virus: uptake and transneuronal passage in the rat central nervous system. J Neurosci. 1990. 10:1974–1994.

12. Card JP, Rinaman L, Lynn RB, Lee BH, Meade RP, Miselis RR, et al. Pseudorabies virus infection of the rat central nervous system: ultrastructural characterization of viral replication, transport, and pathogenesis. J Neurosci. 1993. 13:2515–2539.

13. de Groat WC, Kruse MN, Vizzard MA, Cheng CL, Araki I, Yoshimura N. Modification of urinary bladder function after spinal cord injury. Adv Neurol. 1997. 72:347–364.
14. de Groat WC, Araki I, Vizzard MA, Yoshiyama M, Yoshimura N, Sugaya K, et al. Developmental and injury induced plasticity in the micturition reflex pathway. Behav Brain Res. 1998. 92:127–140.

15. Weiss ML, Chowdhury SI. The renal afferent pathways in the rat: a pseudorabies virus study. Brain Res. 1998. 812:227–241.
16. Nadelhaft I, Booth AM. The location and morphology of preganglionic neurons and the distribution of visceral afferents from the rat pelvic nerve: a horseradish peroxidase study. J Comp Neurol. 1984. 226:238–245.

17. Nadelhaft I, Vera PL. Central nervous system neurons infected by pseudorabies virus injected into the rat urinary bladder following unilateral transection of the pelvic nerve. J Comp Neurol. 1995. 359:443–456.

18. Birder LA, Roppolo JR, Erickson VL, de Groat WC. Increased c-fos expression in spinal lumbosacral projection neurons and preganglionic neurons after irritation of the lower urinary tract in the rat. Brain Res. 1999. 834:55–65.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download