Abstract
Purpose
Itopride hydrochloride (itopride) inhibits acetylcholinesterase (AChE) and antagonizes dopamine D2 receptor, and has been used as a gastroprokinetic agent. However, its prokinetic effect on the small bowel or colon has not yet been thoroughly investigated. The aim of this study was to investigate the effects of itopride on motor functions of the ileum and colon in guinea pigs.
Materials and Methods
The distal ileum was excised and the activity of peristaltic contraction was determined by measuring the amplitude and propagation velocity of peristaltic contraction. The distal colon was removed and connected to the chamber containing Krebs-Henseleit solution (K-H solution). Artificial fecal matter was inserted into the oral side of the lumen, and moved toward the anal side by intraluminal perfusion via peristaltic pump. Colonic transit times were measured by the time required for the artificial feces to move a total length of 10 cm with 2-cm intervals.
Results
In the ileum, itopride accelerated peristaltic velocity at higher dosage (10-10-10-6 M) whereas neostigmine accelerated it only with a lower dosage (10-10-10-9 M). Dopamine (10-8 M) decelerated the velocity that was recovered by itopride infusion. Itopride and neostigmine significantly shortened colonic transit at a higher dosage (10-10-10-6 M). Dopamine (10-8 M) delayed colonic transit time that was also recovered after infusion of itopride.
Gastrointestinal motility is regulated by the enteric nervous system, which is part of the autonomic nervous system, as well as humoral substances.1 Acetylcholine (ACh) from the parasympathetic nerve has an excitatory effect on ileocolonic motility. On the other hand, norepinephrine inhibits the release of AChE from motor neurons, resulting in smooth muscle relaxation.2 Dopamine has also been confirmed as 1 of the enteric neurotransmitters.3 The 2 classical population of dopamine receptors, D1 and D2, are present in the gut. D1 receptors are located mainly on post-junctional effector cells whereas D2 receptors are located on both post- and pre-junctional cells. The pre-junctional D2 receptor exerts a negative modulatory effect on the release of ACh from the intrinsic cholinergic nerve terminal.4,5
Itopride has a benzamide structure and has been known to be a gastroprokinetic agent.6 Itopride stimulates the release of endogenous acetylcholine release by antagonizing dopamine D2 receptor on post-synaptic cholinergic neurons, and also has an anticholinesterase activity, making ACh accumulate at cholinergic receptor sites.7
Itopride has been known to improve symptoms in patients with functional dyspepsia, characterized by early satiety, postprandial fullness, bloating, and nausea.8,9 Several studies reported that itopride enhances gastric emptying in dogs, rats, and humans,10 therefore itopride may be useful for the treatment of functional gastrointestinal disorders. However, the effect of itopride on the small bowel or colonic motility has not been clearly investigated. Therefore, we investigated the effect of itopride on ileal peristalsis and colonic transit in guinea pigs to provide experimental backgrounds for the future application to human functional disorders.
Male guinea pigs of Hartley strain (Nihon SLC Inc., Shizuoka, Japan), weighing approximately 300 g,were individually housed at 22 - 24℃. Guinea pigs were killed by blow to the occipital region in the head and severing the carotid arteries. The experimental procedures were conducted in accordance with the guidelines of Yonsei University Animal Care and Use Committee.
Drugs used in this study included itopride hydrochloride (Abbott, Katsuyama, Japan), dopamine (Sigma, St. Louis, MO, USA), neostigmine (Sigma, St. Louis, MO, USA), and acetylcholine (Sigma, St. Louis, MO, USA).
BIOPAC TSD 105 (BIOPAC System Inc., Santa Barbara, CA, USA) was used to estimate the tension of the circular muscle, and BIOPAC MP 100 (BIOPAC System Inc., Santa Barbara, CA, USA) was used to analyze the data. A peristaltic pump (Masterflex 7523 - 30 with cartridge 3519 - 85, Cole-Palmer, Chicago, IL, USA) was used to induce peristaltic contraction wave.
The method designed by Tonini et al,11 subsequently modified,12 was experimental bath for the estimation of peristalsis. The abdominal cavity was opened and the distal ileum was excised 15 cm from the ileocecal junction. The mesenteric attachment was trimmed away, flushed clean with K-H solution and placed immediately into a bath containing K-H solution. The solution was saturated with 95% O2 and 5% CO2 and maintained at 37℃. The mechanical activity of the circular muscle in the fixed guinea pig ileum was monitored using 3 small clips, arranged at 2.5 cm intervals. The clips were attached via 3 sites of the serosal surface to the underlying circular muscle of the ileum in the peristaltic chamber. These were also attached to independent tension transducers via thread. Initial tension was routinely set to 1 g. Tissues were left to equilibrate in K-H solution for approximately 60 min prior to the start of the experiment. K-H solution was pumped (0.4 mL/min) into the lumen of the ileal segment for 20 min through the peristaltic pump to induce the peristalsis. Itopride (10-10-10-6 M) was added to the bath solution and ileal lumen for 20 min without washing between successive change of concentration. The activity of the peristaltic contraction was determined by amplitude and measurement of the propagation velocity of peristaltic contractions. The propagation velocity was measured by dividing the distance from the oral to the anal side (5 cm) by the time (sec) when the first peristaltic wave moved from the oral to anal side.
To evaluate the mechanism of itopride's action, additional experiments were performed in the presence of neostigmine (10-10-10-7 M), dopamine (10-8 M), and ACh (10-8 M) as previously described. Finally, itopride (10-7 M) was separately administered after dopamine (10-8 M) and ACh (10-8 M) infusion.
The colonic transit study was performed as previously described.13 The distal colon (approximately 10 cm from the anus) was excised, flushed clean with K-H solution, and immediately placed into the bath containing K-H solution saturated with 95% O2 and 5% CO2, at 37℃. Both ends of the colon were connected to the 2 sides (oral and anal) in the chamber. After stabilization for about 60 min, artificial feces (length, 10 mm; width, 4 mm) were inserted into the oral side of the lumen, and K-H solution was pumped (0.4 mL/min) into the lumen of the colon. The artificial feces could then be moved from the oral to the anal side of the colon. Total 10 cm was observed, and the time taken to move about 2 cm was measured. Immediately after the control test, itopride (10-10-10-6 M) was applied to the bath and the lumen by increasing the concentrations without washing between concentrations. For each sample, the time taken to move 2 cm was measured and the last 4 measurements were selected by value of means. Additional experiments were performed in the presence of neostigmine (10-10-10-7 M), dopamine (10-8 M), and ACh (10-8M) as previously described. Finally, itopride (10-7 M) was separately administered after dopamine (10-8 M) and ACh (10-8 M) infusion.
The result for each variable was represented as a percentage of the measurements before administration of itopride (% change of control) and recorded as the mean with standard error. Wilcoxon signed rank test was used for statistical analysis, and the significance level was set at p < 0.05.
Itopride (10-10-10-6 M) infusion in guinea pig ileum increased the amplitude of peristaltic contraction, which was not statistically significant (Fig. 1). Dopamine (10-8 M) alone decreased but itopride (10-7 M) infused after dopamine increased the amplitude, however, with no statistical significance (Fig. 2). Additional itopride (10-7 M) added to ACh (10-8 M) further increased the amplitude, but with no statistical significance (Fig. 3).
Itopride (10-10-10-6 M) significantly accelerated the propagation velocity of the peristalsis (Fig. 4) (p < 0.05). Dopamine (10-8 M) decelerated the propagation velocity to 81.3 ± 5.4%, but itopride (10-7 M) overcame the velocity up to 109.3 ± 3.86% (Fig. 5) (p < 0.05). Neostigmine at a lower concentration (10-10-10-9 M), significantly accelerated the velocity, but it decreased at a higher concentration (10-8-10-7 M) (Fig. 6) (p < 0.05).
Itopride (10-10-10-6 M) significantly dose-dependently shortened colonic transit time compared with the control (Fig. 7) (p < 0.05). Neostigmine (10-10-10-7 M) shortened colonic transit time compared with the control, but was statistically significant only at a higher concentration (10-8-10-7 M) (Fig. 8) (p < 0.05).
Itopride is an AChE inhibitor as well as dopamine D2 receptor antagonist.6,7 It has been known to enhance gastric motility and is used as a treatment for functional dyspepsia.8 Since the M3 muscarinic receptor exists on the smooth muscle layer throughout the whole gut and ACh released from the enteric nerve endings stimulates the contraction of smooth muscle, we believe that itopride has a prokinetic effect on the lower gastrointestinal tract. Indeed, we found that itopride accelerated the velocity of peristaltic contraction of the ileum as well as shortened colonic transit time of guinea pig in vitro.
In early studies, itopride had been known to stimulate only the upper gut.7 Itopride significantly increased the contractile force of only the gastric antrum and duodenum of conscious dogs. In the same study, itopride restored dopamine-induced inhibition of contractions in the gastric antrum and duodenum and the gastroduodenal responses to ACh were enhanced by itopride and neostigmine. In their following studies, the IC50 of itopride for guinea pig gastric AChE inhibition was 50 times as large as that of neostigmine.14 Therefore these authors considered that itopride stimulates endogenous ACh release by blocking dopamine D2 receptor and accumulates endogenous ACh by anticholinesterase activity.
However there are a few in vitro studies to determine the stimulatory effect in the lower gut.15,16 Iwanaga et al.16 compared itopride and physostigmine on contractile responses to ACh and carbachol and reported that itopride increased the amplitude of longitudinal muscle contractions and frequency of peristalsis in an isolated segment in guinea pig ileum. Because itopride enhanced only contractions induced by ACh and failed to increase spontaneous or stimulation-induced ACh release, they concluded that itopride stimulates ileal activity by prevention of ACh hydrolysis.16 In this study, the increase of the amplitude was demonstrated after administration of itopride with ACh, in concordance with previous studies on the increase of contractility.
The action of itopride on colonic motor activity was compared with that of cisapride and mosapride. Many selective 5-HT4 receptor agonists, such as cisapride, mosapride, zacopride, and tegaserod, have been demonstrated to have prokinetic effects on the colon.13,17 In the isolated guinea pig colon, only itopride significantly increased the frequency of peristaltic and segmental contractions in the proximal and distal colon without changing amplitude,15 which is partly consistent with our data. Colonic transit in guinea pigs and rats was also accelerated significantly by itopride but not by cisapride or mosapride. In conscious dogs, itopride showed stimulatory effect on digestive organs, ranging from the stomach to the colon. They thought that the effect of itopride on the colon is mainly due to anti-AChE activity because gastrointestinal smooth muscle is directly stimulated by ACh through the activation of the M3 muscarinic receptor. However in our study, dopamine decreased the propagation velocity of the ileum and significantly delayed the colonic transit time, which were counter-acted by the administration of itopride. It is highly likely that blocking effect of itopride on dopamine D2 receptor also modulates colonic motility. To our dismay, the administration of ACh after itopride did not exhibit a positive additive effect, and further investigation is needed to prove whether a cumulative effect indeed exists.
Similar to other studies on itopride,7,16 we evaluated the AChE activity of itopride on the ileum and colon and found that neostigmine accelerated the propagation velocity of the ileum at lower concentrations and dose-dependently shortened the colonic transit time. This pattern was similar to those obtained with itopride, except at higher concentrations, in the ileum. This concentration dependent effect is due to neostigmine's narrow concentration window.18 The accumulation of ACh at intraganglionic synapses or neuromuscular junctions by neostigmine does not effectively facilitate coordinated peristalsis but inhibits peristalsis at higher concentrations.18
In conclusion, itopride has a stimulatory effect on the ileum and colon, and this is mediated through dopamine D2 receptor antagonist and AChE inhibitory action. These data will provide experimental background for the use of itopride to treat patients with constipation or other functional bowel disorders.
Figures and Tables
Fig. 1
The effect of itopride on amplitude of peristaltic contraction. Itopride (10-10-10-6 M) did not change the amplitude of peristaltic contraction significantly.

Fig. 2
The effect of dopamine alone and itopride plus dopamine on amplitude of peristaltic contraction. Dopamine (10-8 M) or dopamine with itopride (10-7 M) did not change the amplitude significantly.
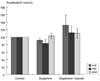
Fig. 3
Itopride (10-7 M) added to ACh (10-8 M) further increased the amplitude with no statistical significance.

Fig. 4
The effect of itopride on the propagation velocity of the peristaltic contraction. Itopride (10-10-10-6 M) significantly accelerated the velocity dose-dependently (*p < 0.05).

Fig. 5
The effect of dopamine alone and dopamine plus itopride on the propagation velocity of the peristaltic contraction. Dopamine (10-8 M) significantly decelerated the propagation velocity of the peristaltic contraction to 81.3 ± 5.4% (*p < 0.05). When itopride (10-7 M) was administered in the presence of dopamine, the velocity was increased up to 109 ± 3.86% with statistical significance (†p < 0.05).
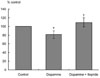
Fig. 6
The effect of neostgmine on the propagation velocity of peristaltic contraction. Neostigmine at lower concentration significantly increased the velocity, but decreased at higher concentrations (*p < 0.05).

Fig. 7
The effect of itopride on the colonic transit time. Itopride (10-10-10-6 M) dose-dependently shortened colonic transit time (*p < 0.05).

Fig. 8
The effect of neostigmine on colonic transit time. Neostigmine (10-10-10-7 M) dose-dependently shortened colonic transit time (*p < 0.05).

References
1. Scratcherd T, Grundy D. The physiology of intestinal motility and secretion. Br J Anaesth. 1984. 56:3–18.

2. Li ZS, Schmauss C, Cuenca A, Ratcliffe E, Gershon MD. Physiological modulation of intestinal motility by enteric dopaminergic neurons and the D2 receptor : analysis of dopamine receptor expression, location, development, and function in wild-type and knock-out mice. J Neurosci. 2006. 26:2798–2807.

3. Tonini M, Cipollina L, Poluzzi E, Cremas F, Corazza GR, De Ponti F. Review article: clinical implications of enteric and central D2 receptor blockade by antidopaminergic gastrointestinal prokinetics. Aliment Pharmacol Ther. 2004. 19:379–390.

4. Tonini M. Recent advances in the pharmacology of gastrointestinal prokinetics. Pharmacol Res. 1996. 33:217–226.

5. Schuurkes JA, Van Nueten JM. Domperidone improves myogenically transmitted antroduodenal coordination by blocking dopaminergic receptor sites. Scand J Gastroenterol Suppl. 1984. 96:101–110.
6. Sakaguchi J, Nishino H, Ogawa N, Iwanaga Y, Yasuda S, Kato H, et al. Synthesis, gastrointestinal prokinetic activity and structure-activity relationships of novel N-[[2-(dialkylamino) ethoxy]benzyl] benzamide derivatives. Chem Pharm Bull(Tokyo). 1992. 40:202–211.

7. Iwanaga Y, Miyashita N, Morikawa K, Mizumoto A, Kondo Y, Itoh Z. A novel water-soluble dopamine-2 antagonist with anticholinesterase activity in gastrointestinal motor activity. Gastroenterology. 1990. 99:401–408.

8. Holtmann G, Talley NJ, Liebregts T, Adam B, Parow C. A placebo-controlled trial of itopride in functional dyspepsia. N Engl J Med. 2006. 354:832–840.

9. Amarapurkar DN, Rane P. Randomised, double-blind, comparative study to evaluate the efficacy and safety of ganaton (itopride hydrochloride) and mosapride citrate in the management of functional dyspepsia. J Indian Med Assoc. 2004. 102:735–737. 760
10. Iwanaga Y, Miyashita N, Saito T, Morikawa K, Itoh Z. Gastroprokinetic effect of a new benzamide derivative itopride and its action mechanisms in conscious dogs. Jpn J Pharmacol. 1996. 71:129–137.

11. Tonini M, Galligan JJ, North RA. Effects of cisapride on cholinergic neurotransmission and propulsive motility in the guinea pig ileum. Gastroenterology. 1989. 96(5 Pt 1):1257–1264.
12. Ji SW, Park H, Chung JP, Lee SI, Lee YH. Effects of tegaserod on ileal peristalsis of guinea pig in vitro. J Pharmacol Sci. 2004. 94:144–152.

13. Ji SW, Park HJ, Cho JS, Lim JH, Lee SI. Investigation into the effects of mosapride on motility of Guinea pig stomach, ileum, and colon. Yonsei Med J. 2003. 44:653–664.
14. Iwanaga Y, Kimura T, Miyashita N, Morikawa K, Nagata O, Itoh Z, et al. Characterization of acetylcholinesterase-inhibition by itopride. Jpn J Pharmacol. 1994. 66:317–322.

15. Tsubouchi T, Saito T, Mizutani F, Yamauchi T, Iwanaga Y. Stimulatory action of itopride hydrochloride on colonic motor activity in vitro and in vivo. J Pharmacol Exp Ther. 2003. 306:787–793.

16. Iwanaga Y, Suzuki N, Kato K, Kimura T, Morikawa K, Kato H, et al. Stimulatory effects of HSR-803 on ileal motor activity. Jpn J Pharmacol. 1993. 62:395–401.

17. Mine Y, Yoshikawa T, Oku S, Nagai R, Yoshida H, Hosoki K. Comparison of effect of mosapride citrate and existing 5-HT4 receptor agonists on gastrointestinal motility in vivo and in vitro. J Pharmacol Exp Ther. 1997. 283:1000–1008.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download