Abstract
Purpose
The present study is to investigate the effects of type 1 diabetes mellitus on dentition and oral health for children and adolescents.
Materials and Methods
The
investigation was carried out on 100 subjects. The first group consisted of 50 subjects with type 1 diabetes mellitus (21 females, 29 males), age 9 ± 0.14 years; In the second group, there were 50 healthy subjects who did not suffer from any systemic disease (25 females, 25 males), age 9 ± 0.11 years. The subjects were evaluated and divided into two groups of 5 - 9 years old, and 10 - 14 years old. The dentition of all participants was examined. Besides, the DFS/dfs index, oral hygiene conditions were evaluated, as well as the plaque index (PI), gingival index (GI) and calculus index (CI). The data obtained from each group were compared statistically.
Results
When compared to the non-diabetic group, we observed that dental development was accelerated until the age of 10 in the diabetic group, and there was a delay after the age of 10. The edentulous interval was longer in the group with type 1 diabetes mellitus. This was accompanied by a high ratio of gingival inflammation. Gingival inflammation was 69.7% in the group of 5 - 9 year-old, and 83.7% in the group of 10 - 14 year-old with type 1 diabetes mellitus. Though there was a greater loss of teeth in the group with type 1 diabetes mellitus, there were more caries in the control group. The PI, GI and CI values showed an increase with aging in favor of the group with type 1 diabetes mellitus. There was statistically significant difference in PI, GI and CI between the control and type 1 diabetes mellitus groups for 10 - 14 year-old patients (p < 0.001).
Diabetes mellitus (DM) is a metabolic disease leading to abnormal fat, carbohydrate, and protein metabolism.1,2 Two basic types of primary diabetes mellitus have been described: insulin-dependent diabetes mellitus (IDDM; type I) and non-insulin-dependent diabetes mellitus (NIDDM; type II).3
The prevalence of type 1 diabetes mellitus exhibits a wide range, as great as 20 - 60 times, in separate geographic populations.4 According to data of World Health Organization DIAMOND Project Group, while the incidence of type 1 diabetes mellitus was low in Asia and South America, it was high in Europe.5 In addition, the incidence rate is currently increasing in Europe.6 The prevalence of Type 1 Diabetes Mellitus was found to be 0.09 per 1000 in Japan and, 1.91 per 1000 in Finland.5,7 It was reported to be 0.27 per 1000 in Turkey.8
The main complications of diabetes mellitus affect the organs and tissues rich in capillary vessels, such as the kidney, retina and nerves. These complications are secondary to the development of microangiopathy.9 Similar changes in small vessels can be found in the oral tissues.10,11 Periodontal disease has also been recognized as the sixth leading complication of diabetes.12 All of these long-term consequences have been widely studied in recent years, and this has led to improvements in disease prevention and effective therapy, thus giving patients with diabetes a better quality of life.6
The influence of diabetes on the risk of developing periodontal disease has been the subject of much discussion in literature. According to a majority of authors, individuals suffering from diabetes mellitus are at an increased risk for developing periodontal disease.13-17 There is, however, a small group of other researchers who have failed to find this increased risk.18-21 Currently, the most widely accepted theory has been offered by Glickman, the father of modern periodontology, who considered diabetes not the direct cause, but a predisposing condition for periodontal disease.22 Metabolic imbalances in the tissues may lower the resistance of diabetics to infection,11 and so influence the initiation, development and progression of periodontal disease.2 Reports on the relationship between dentition and diabetic disease of young diabetics are few, and the results have been variable.23,24
The aim of this study was to determine whether or not there was any change in the dentition of patients with Type 1 Diabetes Mellitus, to assess the rate of gingival inflammation accompanying dentition, and to contribute to the incidence data on periodontal disease for groups of patients where factors attributable to aging are not confounding variables.
The study group consisted of a sample of 50 children and adolescents, ranging in age from 6 - 14 years who were referred to us for treatment of their poorly controlled type 1 diabetes mellitus at the Ataturk University Pediatric-Diabetes Clinic in Erzurum, Turkey. In the second (control) group, there were 50 young subjects who were not suffering from a systemic disease. All the subjects were of similar age, gender, socioeconomic and social class distribution. The subjects were evaluated and then divided into two groups of age 5 - 9, and 10 - 14 year-old. The children and their family were informed about the method and purpose of the study, and they consented to regular dental follow-up examinations every third month during the 1-year period. Data on blood glucose and glycosylated hemoglobin (HbA1c) were collected from the medical records. The age of the patient and the metabolic control of their diabetes were provided through medical anamnesis. Subjects were excluded for the following criteri: the use of a drug that would affect the mouth flora, immune system or the inflammatory response; a periodontal treatment in the last 6 months prior to the assessment; and the presence of any severe pathology of the teeth.
Dentition was the first thing we looked for on the dental examination. The overall health of the erupted and erupting teeth and the tissues surrounding these teeth was examined and four bite-wing radiographs were exposed. The health of the surrounding tissues was examined for the main symptoms inflammation (rubor, colour, dolor, tumor). If at least one of these was present, the gingival inflammation symptoms were assessed as "existing" or "non-existing". If the tissues surrounding the erupted tooth had at least one of the main symptoms of inflammation (rubor, colour, dolor, tumor), it is assessed as "existing". The evaluations were made at the Department of Pedodonti, Ataturk University, by an experienced colleague (pedodontist). Cases of manifest caries (DFS/dfs) were scored according to the examination protocol that has been advocated by the World Health Organization (WHO).25 In addition, manifest and initial interdental lesions on the radiographs were assessed according to the guidelines of Grondahl et al.26 The level of oral hygiene was estimated with a plaque index,27 gingival index28 and calculus index.29 These evaluations were made in the Department of Periodontology, Ataturk University, by the same periodontist.
Concerning medical check-ups at the hospital, all subjects saw a periodontist every third month during the 1-year period. The same procedures were also conducted for the healthy group. At each visit, the level of oral hygiene was assessed. Caries recordings with bit-wing radiographs were repeated annually. Based on individual need, oral hygiene instructions and professional teeth cleaning with interdental flossing were provided. Each visit was finished with fluoride varnish applications on all surfaces after gentle drying with air and cotton rolls.
The plaque index scores were recorded for 4 tooth surfaces (mesial, distal, buccal, and lingual) and the quantity of plaque was assessed at the cervical area of every tooth (Table 1). The gingival index was recorded for the mesial, distal, buccal, and lingual surfaces with a manual periodontal probe (Williams' periodontal probe designed with 1, 2, 3, 5, 7, 9, and 10 millimeter calibrations) on the gingival area adjacent to the teeth in each patient. Bleeding was recorded if it occurred within 30 s of probing (Table 2). The calculus index scores were recorded on the four tooth surfaces (mesial, distal, buccal, and lingual), and the quantity of calculus was assessed at the cervical area of every tooth (Table 3). The numerical scores of the plaque index and gingival index were calculated according to the formula: Per person = sum of individual scores/number of teeth present for each person, and subsequently, the group score was obtained.
At the end of the examination each patient received advice, a dental prophylaxis, and was offered urgent dental care.
The investigation was carried out on 100 subjects. The first group (study group) consisted of 50 subjects with type 1 diabetes mellitus (21 females, 29 males), age 9 ± 0.14 years; In the second group (control group), there were 50 healthy subjects who did not suffer from a systemic disease (25 females, 25 males), and their ages were 9 ± 0.11 years. Some of the clinical characteristics are given in Table 4. The HbA1c, glucose, and BUN in the diabetics were higher than in the non-diabetics (p < 0.001).
The distribution of erupted teeth in the group with type 1 diabetes mellitus according to the age is given in Table 5, and that of the control group is given in Table 6.
When comparing the diabetic group with the non-diabetic group, we observed that the dental development of diabetic group was fast until the age of 10, and then dental development decreased after the age of 10. In addition, the edentulous interval was longer in the type 1 diabetes mellitus group.
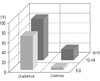
Gingival inflammation in the individuals with poor metabolic control was accompanied by a higher ratio of eruption. The accompanying percentage of gingival inflammation to eruption in the group of 5 - 9 year-old diabetics was 69.7%, while it was 5.2% for the control group of the same age. This percentage in the diabetics group of 10 - 14 year olds was 83.7%, and it was 21.4% in the control group (Fig. 1).
The incidence of caries in non-diabetics was less than for type 1 diabetes mellitus subjects, and this data is presented in Table 7. The subjects with poor metabolic control already had slightly more caries at the baseline, although the difference was not statistically significant (p > 0.05). The incidence of caries in permanent dentition was higher in the group with poor metabolic control (p < 0.05). The amount of caries was noted to increase with age for both the diabetic and non-diabetic groups.
When compared with individuals of the same age group, we observed an increase in periodontal diseases in the Type 1 Diabetes Mellitus group (Table 8).
At the beginning, the PI in diabetic patients 5 - 9 year-old was 1.47 ± 0.4, and it was 1.15 ± 0.4 in the control group. The difference between them was also statistically insignificant (p < 0.01). In contrast to these groups, the PI for the diabetics in the 10 - 14 year-old group was 1.92 ± 0.6, and it was 1.21 ± 0.4 in the control group, and the difference between them was statistically significant (p < 0.001). A similar relation in gingival index increase in the diabetic group was seen. The GI in the 5 - 9 year-old diabetic group was 1.54 ± 0.5, and it was 1.14 ± 0.5 in the control group. The difference between them was statistically significant (p < 0.01). The GI in the 10 - 14 year-old group with diabetics was 1.98 ± 0.6, while it was 1.17 ± 0.5 in control group, and the difference was statistically significant (p < 0.001). It was found that amount of calculus increased with ageing in both groups. Although there was no difference in calculus index in the 5 - 9 year-old diabetic and non-diabetic groups, it increased in the 10 - 14 year-old diabetic group; it was 1.61 ± 0.3 in the diabetic group, and 0.64 ± 0.4 in the control group. The difference between them was statistically significant (p < 0.001).
After 1-year, the PI in the diabetic 5 to 9 year-old group was 1.79 ± 0.1, and it was 0.97 ± 0.2 in the control group (Table 9). The difference between them was also statistically insignificant (p < 0.001). In contrast to these groups, the PI in the diabetic 10 - 14 year-old was 1.99 ± 0.6, and it was 1.02 ± 0.2 in the control group, and the difference between them was statistically significant (p < 0.001). A similar relation for the gingival index increase in the diabetic group was seen. The GI in the 5 - 9 year-old group with diabetics was 1.60 ± 0.3, and it was 0.97 ± 0.2 in control group. The difference between them was significant statistically significant (p < 0.001). The GI in the 10 - 14 year-old group with diabetics was 2.01 ± 0.6, while it was 1.19 ± 0.3 in the control group. The difference was statistically significant (p < 0.001). It was found that the calculus amount increased with ageing in both groups. Although there was no difference for the calculus index in the 5 - 9 year-old diabetic and non-diabetic groups, it increased in the 10 - 14 year-old diabetic group. It was 1.75 ± 0.6 in diabetic group, and 0.79 ± 0.6 in control group. The difference between them was statistically significant (p < 0.001).
Children with diabetes endure many problems during the course their lives. Dentition and oral health problems are among these. In this study, the effects of diabetes on dentition and oral health were evaluated by using clinical findings.
Eruption is the cutting of the tooth through oral mucosa, and it is seen in the mouth by the upright action of the tooth through the crista of the gums. It is a regular physiological process of growth and no inflammation occurs under normal conditions. However, it has been shown that diseases containing metabolic instabilities like diabetics weaken the resistance to inflammation in individuals.11 Indeed, in our study, gingival inflammation accompanied eruption in the diabetic individuals at a higher rate than in the non- diabetic ones.
Especially, there is limited information available in the 5 - 9 age group on the dentition of the patients with Type 1 Diabetes Mellitus. Based on a study of about 60 children with diabetes, Ziskin et al.23 reported an insignificant influence of diabetes on dental development. Bohatka et al.24 reported accelerated dental development in diabetic children less than 11.5 years old; older diabetic children manifested delayed dental development. Adler et al.,30 have suggested that metabolic disorders as responsible for the acceleration and delay seen in dentition. Our study results are compatible with that of Bohatka's. Yet, in our study, acceleration in dentition until the age of 10, and a delay after the age of 10 were observed. This result is incongruent with common knowledge (especially for the eruption of canine and premolars). In our present study, an acceleration in dentition until the age of 10, and a delay after the age of 10 may be related to the diseases like diabetes containing metabolical instabilities.
It was suggested by some authors that salivary secretion rates may be significantly reduced in children with Type 1 Diabetes Mellitus when compared to healthy children.31 Reduced salivary secretion increases the likelihood of caries, but good metabolic control prevents the most dangerous salivary changes such as high glucose content and lower pH, while a good diabetic diet, rich in fiber and low in simple carbohydrates, can slow down the production of plaque and the proliferation of acidogenic bacterial microflora.32,33 In the present study, the amount of caries increased with age both in diabetic and non-diabetic groups. In addition, it has been demonstrated that children with Type 1 Diabetes Mellitus have less caries than non-diabetic children in all the age groups. However, the investigation we carried out showed that this situation resulted from an abundance of sites where teeth were lost and not replaced. To our knowledge, these complications were not symptoms of Type 1 Diabetes Mellitus; they may be due to poor medical and dental care.
Although gingival bleeding is an indicator of inflammation, it is possible that vascular changes in diabetes mellitus result in increased gingival bleeding. Tchobroutsky34 observed fewer vascular changes in well controlled diabetes. In the present study, the gingival index scores were found to be higher in diabetic children than those of non-diabetics.
Gingival bleeding has a positive correlation with the accumulation of plaque and calculus.22 Plaque and calculus deposits (which are the most important pathogenic factors of periodontopathy) should be removed either with ultrasound or with hand instruments. Our results showed that there was more plaque and higher calculus index scores in diabetics than in the control groups. Calcium concentration was increased in both the parotid and submandibular saliva of patients with IDDM,35 a fact that may explain the frequently reported increase in calculus formation in these patients.8,36-38 Sheridan et al.39 have shown that pocket formation, the presence of calculus, increased tooth mobility and tooth loss occurred with greater frequency in patients with a decreased glucose tolerance.
There is limited information regarding the effectiveness of periodontal disease treatment on the metabolic control of diabetes mellitus. It may be that chronic Gram-negative infections and chronic endotoxaemia, such as occur for periodontal disease, could also induce insulin resistance and worsening of metabolic control in diabetic patient, and thus, this provides the basis for the hypothesis that elimination or control of periodontal infection may improve metabolic control of diabetes.40 In one small study, insulin requirements were reduced following periodontal treatment and reduction of inflammation in diabetic patients.41 A pilot study reported the reduction in levels of glycosylated hemoglobin in five of nine patients after 8 weeks of periodontal treatment.42
The prevention of periodontal breakdown in diabetic patients is mostly based on the education of the individual. Thus, patients should be informed about the importance of oral health for diabetics, and they should be taught that the main symptom of periodontal disease is gingival bleeding.6 Plaque and calculus deposits, which are the most important pathogenic factors of periodontopathy in the oral cavity, should be removed through careful self-care and regular professional care to reduce the risk of periodontitis for diabetics. Patients should also learn how to brush their teeth correctly, which should be done at least twice a day, and how to use dental floss and sometimes chlorhexidine digluconate 0.2%.
In summery, the results suggest that Type 1 Diabetes Mellitus plays a significant role for dentition and oral health in children and adolescents. The children with Type 1 Diabetes Mellitus are more likely to experience infections in connective tissues than children without Type 1 Diabetes Mellitus. This is due to the fact that for children with Type 1 Diabetes Mellitus, infection leads to loss of teeth. However, in children without Type 1 Diabetes Mellitus, the teeth were more likely to remain viable. The pediatrician's concern is to maintain good metabolic control and to make diabetic patients aware of a diet that suits their unique nutritional needs. The obligation of the dentist to the patient is to evaluate and help maintain good oral hygiene.
Figures and Tables
Table 7
The Prevelence of Caries for Subjects with Type 1 Diabetes Mellitus Subjects and Healthy Control Subjects

References
1. Becker DJ. Lifshitz F, editor. Diabetes mellitus and hypoglycemia. Pediatric Endocrinology. 1996. 3rd ed. New York: Marcel Dekker, Inc;555–566.
2. Ervasti T, Knuuttila M, Pohjamo L, Haukipuro K. Relation between control of diabetes and gingival bleeding. J Periodontol. 1985. 56:154–157.

3. Karam JH. Greenspan FS, Strewler GJ, editors. Pancreatic Hormones and diabetes mellitus. Basic and Clinical Endocrinology. 1997. 5th ed. New Jersey: Appleton & Lange;595–663.
4. WHO Study Group Report. Prevention of Diabetes Mellitus. 1994. Geneva: World Health Organization;WHO Technical Report series no.844.
5. Karvonen M, Tuomilehto J, Libman I, LaPorte R. A review of the recent epidemiological data on the worldwide incidence of type 1 (insulin-dependent) diabetes mellitus. World Health Organization DIAMOND Project Group. Diabetologia. 1993. 36:883–892.

6. Iughetti L, Marino R, Bertolani MF, Bernasconi S. Oral health in children and adolescents with IDDM-a review. J Pediatr Endocrinol Metab. 1999. 12:603–610.
7. Diabetes Epidemiology Research International Group. Geographic patterns of childhood insulin-dependent diabetes mellitus. Diabetes. 1988. 37:1113–1119.
8. Hatun S, Tezic T. Ankaradaki okul cocuklarinda insuline bagimli diabetes mellitus prevalansi. Cocuk Sagligive Hastaliklari Dergisi. 1996. 39:465–471.
9. Hanssen KF. Blood glucose control and microvascular and macrovascular complications in diabetes. Diabetes. 1997. 46:Suppl 2. S101–S103.

10. Listgarten MA, Ricker FH Jr, Laster L, Shapiro J, Cohen DW. Vascular basement lamina thickness in the normal and inflamed gingiva of diabetics and non-diabetics. J Periodontol. 1974. 45:676–684.

11. Frantzis TG, Reeve CM, Brown AL Jr. The ultrastructure of capillary basement membranes in the attached gingiva of diabetics and nondiabetics patients with periodontal disease. J Periodontol. 1971. 42:406–411.

12. Grossi SG, Zambon JJ, Ho AW, Koch G, Dunford RG, Machtei EE, et al. Assessment of risk for periodontal disease. I. Risk indicators for attachment loss. J Periodontol. 1994. 65:260–267.

13. Miotti F, Ferro R, Saran G. Diabetes in oral medicine (Current status of knowledge, diagnosis, therapy and dental prevention). G Stomatol Ortognatodonzia. 1985. 4:3–14.
14. Finestone AJ, Boorujy SR. Diabetes mellitus and periodontal disease. Diabetes. 1967. 16:336–340.

15. Sznajder N, Carraro JJ, Rugna S, Sereday M. Periodontal findings in diabetic and nondiabetics patients. J Periodontol. 1978. 49:445–448.
16. Cianciola LJ, Park BH, Bruck E, Mosovich L, Genco RJ. Prevalence of periodontal disease in insulin-dependent diabetes mellitus (juvenile diabetes). J Am Dent Assoc. 1982. 104:653–660.

17. Hugoson A, Thorstensson H, Falk H, Kuylenstierna J. Periodontal conditions in insulin-dependent diabetics. J Clin Periodontol. 1989. 16:215–223.

18. Benveniste R, Bixler D, Conneally PM. Periodontal disease in diabetics. J Periodontol. 1967. 38:271–279.

20. Bay I, Ainamo J, Gad T. The response of young diabetics to periodontal treatment. J Periodontol. 1974. 45:806–808.

21. Barnett ML, Baker RL, Yancey JM, MacMillan DR, Kotoyan M. Absence of periodontitis in a population of insulin-dependent diabetes mellitus (IDDM) patients. J Periodontol. 1984. 55:402–405.

22. Carranza FA, Newman MG. Irving Glickman's Clinical Periodontology Glickman. Clinical periodontology. 1996. 8th ed. Philadelphia: WB Saunders Co;281–297.
23. Ziskin DE, Siegel EH, Loughlin WC. Diabetes in relation to certain oral and systemic problems. Part I: clinical study of dental caries, tooth eruption, gingival changes, growth phenomena and related observations in juveniles. J Dent Res. 1944. 23:317–331.

24. Bohátka L, Wegner H, Adler P. Parameters of the mixed dentition in diabetic children. J Dent Res. 1973. 52:131–135.

25. WHO. Oral health surveys-basic methods. 1987. 3rd ed. Geneva:
26. Gröndahl HG, Hollender L, Malmcrona E, Sundquist B. Dental caries and restorations in teenagers. I. Index and score system for radiographic studies of proximal surfaces. Swed Dent J. 1997. 1:45–50.
27. Silness J, Löe H. Periodontal disease in pregnancy II. Correlation between oral hygiene and periodontal condition. Acta Odontol Scand. 1964. 24:121–135.
28. Löe H, Silness J. Periodontal disease in pregnancy I. Prevalence and severity. Acta Odontol Scand. 1963. 21:533–551.

30. Adler P, Wegner H, Bohatka L. Influence of age and duration of diabetes on dental development in diabetic children. J Dent Res. 1973. 52:535–537.

31. Ben-Aryeh H, Serouya R, Kanter Y, Szargel R, Laufer D. Oral health and salivary composition in diabetic patients. J Diabetes Complications. 1993. 7:57–62.

32. Karjalainen KM, Knuuttila ML, Käär ML. Relationship between caries and level of metabolic balance in children and adolescents with insulin-dependent diabetes mellitus. Caries Res. 1997. 31:13–18.

33. Got I, Fontaine A. Teeth and diabetes. Diabete Metab. 1993. 19:467–471.
34. Tchobroutsky G. Relation of diabetic control to development of microvascular complications. Diabetologia. 1978. 15:143–152.

35. Goteiner D, Vogel R, Deasy M, Goteiner C. Periodontal and caries experience in children with insulin-dependent diabetes mellitus. J Am Dent Assoc. 1986. 113:277–279.

36. Swanljung O, Meurman JH, Torkko H, Sandholm L, Kaprio E, Mäenpää J. Caries and saliva in 12-18 year old-diabetics and controls. Scand J Dent Res. 1992. 100:310–313.
37. Karjalainen KM, Knuuttila ML, Käär ML. Relationship between caries and level of metabolic balance in children and adolescents with insulin-dependent diabetes mellitus. Caries Res. 1997. 31:13–18.
38. Wegner H. Dental caries in young diabetics. Caries Res. 1971. 5:188–192.
39. Sheridan RC Jr, Cheraskin E, Flyn AC. Epidemiology of diabetes mellitus II. 100 dental patients. J Periodontol. 1959. 30:298–323.
40. van Adrichem LN, Hovius SE, van Strick R, van der Meulen JC. Acute effects of cigarette smoking on the microcirculation of the thumb. Br J Plast Surg. 1992. 45:9–11.




 PDF
PDF ePub
ePub Citation
Citation Print
Print










 XML Download
XML Download