Abstract
Various methods have been used to investigate the hair shaft. In the ultrastructural hair field, scanning and transmission electron microscopies are widely used investigative methods, but they have some technical limitations. Recently, X-ray microscopes with sub-micron spatial resolution have emerged as useful instruments because they offer a unique opportunity to observe the interior of an undamaged sample in greater detail. In this report, we examined damaged hair shaft tips using hard X-ray microscopy with a 90 nm spatial resolution. The results of this study suggest that hard X-ray microscopy is an alternative investigative method for hair morphology studies.
Various methods have been employed for investigating the hair shaft such as visual inspection, detection of physical properties, such as tensile properties, torsional properties, and chemical properties of hair, especially protein. For the ultrastructural study of hair, scanning and transmission electron microscopies have been used widely. Although they are efficient and basic methods of investigating hair, they have several limitations.1 Transmission electron microscopy (TEM) requires sophisticated processes including drying, dyeing, and cutting, after which, it can show only a transverse section of internal hair structure. Scanning electron microscopy (SEM) requires less sophisticated preparation than TEM, but its images are limited to the surface of hair structure.
Worldwide interest in X-ray microscopes with sub-micron spatial resolution has been increasing. In the life sciences, successful progress has been made by using soft X-ray microscopy and its electromagnetic spectrum. Using this instrument, researchers have been able to achieve satisfactory spatial resolution.2 However, due to the short depth of focus and the limited penetration of the hard X-ray, there has been demand for a hard X-ray technique that can penetrate thicker samples. Here, we introduce the optical system of our microscope and present hair images taken by a hard X-ray microscope. In comparing the traditional methods such as electron microscopes with our hard X-ray procedure, we found that this new technique offers unique opportunities to examine the internal structures of a sample in detail without damaging it.
For this study, three healthy individuals (22, 28, and 32-year-old females) were selected. The 22-year-old female had not experienced any factors that might have induced extrinsic hair damage during the six months prior to this study. The 28-year-old and 32-year-old females had their hair dyed more than two weeks prior to the collection of their hair samples. Hair samples were taken from the occipital area, and the distal portion of the hair was selected to inspect the degree of damage and was mounted on the sample plate for hard X-ray microscopic study.
The Zernlike-type phase contrast hard X-ray microscope was set on a 1B2 beam line of PLS (Pohang Light Source), which is a third generation synchrotron radiation facility with the operating energy of 2.5 GeV at Pohang, Korea (Fig. 1). The X-ray beam was generated by a bending magnet that was monochromatized by a silicon (111) channel-cut crystal monochromator. The beam was then focused through a 4-mm diameter condenser zone plate (CZP) and onto the sample where the X-ray image of the sample was magnified 32.5 times by an objective (micro) zone plate. The outer and the inner diameter of the condenser zone plate was 4 and 1.5 mm, respectively. The diameter of the objective zone plate was 160 microns. Because a hair shaft is transparent to hard X-rays with an energy of 7 keV (= 1.78 A), an X-ray version of the Zernike phase contrast using visible light microscopy was realized with an annular aperture and a phase plate. The magnified X-ray image of a sample was converted into a visual one on the CsI (T1) scintillation crystal. This visual image was further enlarged 20 times with a microscopic objective lens and captured by a full frame charge-coupled device(CCD) camera.
Fig. 2 shows the images obtained by hard X-ray. Fig. 2A, 2B, and 2C represent pictures from the hard X-ray microscope. The images were made by combining multiple smaller images in order to accommodate the restricted field of view. Fig. 2A is a natural hair tip image from the 22-year-old female volunteer's sample. It shows a round tip at the end of the hair. Fig. 2B is a damaged hair tip image from the 28-year-old female volunteer's sample. The irregularly shaped hair tip might be caused by physical and chemical damages during the hair dyeing process. Fig. 2C is a damaged hair tip image from the 32-year-old female volunteer's sample. Here, we noticed extrinsic damage caused by a cosmetic procedure.
The most commonly used ultrastructural instrument to study morphological changes of hair is the electron microscope. By using an electron microscope, it is possible to take images of a sample shortly after several required processes. There have been some attempts to find another investigative method for morphological studies of hair, and the assessment of human hair by confocal microscopy seems to be an example of those efforts.3 Recently, several reports were published using X-ray diffraction for a detailed study of the keratinization process in a human hair follicle, structural changes in hair tissue due to the influence of exogenous factors,4 and the early diagnosis of breast cancer, although its diagnostic accuracy has been debated.5,6 Moreover, conventional X-rays have been used widely in medical applications such as diagnostic imaging.
Synchrotron X-rays can produce a superior image quality, which is characterized by high resolution and coherence-based edge enhancement. Because of its high intensity, tunable energy, and polarization of the synchrotron radiation, the use of hard X-rays can be an useful source for various tools in the medical field of structural research.7 Meanwhile, microscopically detailed images of moving blood vessels in living animals without the use of contrast agents have been captured through synchrotron microangiography.8
The important advantage of X-ray microscopes over electron microscopes is their ability to observe the inside structure of samples without causing inevitable damage to them. In the soft X-ray region of the electromagnetic spectrum, a spatial resolution around 20 nm has already been achieved,2 and the natural contrast of the so-called water window with the spatial resolution has enabled the soft X-ray microscope to be a successful device to study bio-samples. However, soft X-ray microscopy has several drawbacks; one is its short depth of focus, and another is its weak penetration. X-ray imaging with multi-KeV photon energy permits penetration of thicker samples that are not accessible to a soft X-ray microscope.9 The diameter of human hair is about 100 micrometers (µm), so it is suitable to examine a sample by using a Hard X-ray microscope. There have been attempts to overcome the shortcomings using hard X-rays, and we have developed a new hard X-ray microscope with a sub-micron spatial resolution at PLS.10,11 Some authors also reported images of extrinsically damaged hair tresses using a hard X-ray microscope, focusing on the differences among the causes of extrinsic damage.12
It is true that images from a Hard X-ray microscope are not superior to EM images in terms of resolution. However, in considering that it is possible to see only the surface structures of hair by SEM, that the sample is often damaged during the preparation step, and that the study of transverse images are possible only through TEM, hard X-ray microscopy could become an attractive investigative method. When considering these strong points, it is worth the effort to observe samples with hair shaft abnormalities using this improved spatial resolution. However, there are still many problems with the use of hard X-rays, including the cost to perform hard X-ray analysis, lower resolution than conventional electron microscopy, and an absence of standardized methods. However, technologies related to X-ray microscopy are being improved quickly, and so these drawbacks may be overcome in the near future.
In summary, we suggest that the hard X-ray microscope is another viable investigative tool in the morphological study of hair, and we believe that it will be possible to obtain a higher quality of images after further technological improvement of the hard X-ray in the near future.
Figures and Tables
Fig. 1
Layout of the optical system in the X-ray microscope. The size of each optical component is not to scale. The diameter of the condenser and the objective zone plate are 4000 and 160 microns, respectively (Adapted from Youn HS and Jung SW, Observations of the human hair shaft with an X-ray microscope, Phy Med Biol 2005;50:5417-20). PLS, Pohang Light Source.
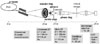
Fig. 2
Hair tip images from a hard X-ray microscope. (A) an image from a 22-year-old female volunteer with a natural hair tip viewed through the hard X-ray microscope. (B) an image from a 28-year-old female volunteer with a weathering hair tip viewed through the hard X-ray microscope. (C) an image from a 32-year-old female volunteer with a weathering hair tip viewed through the hard X-ray microscope. The exposure time was 60 seconds for all samples (bar 50 µm).

References
1. Wolfram LJ. Human hair: A unique physicochemical composite. J Am Acad Dermatol. 2003. 48:Suppl 1. S106–S114.

2. Chao W, Anderson E, Denbeaux GP, Harteneck B, Liddle JA, Olynick DL, et al. 20-nm resolution x-ray microscopy demonstrated by use of multilayer test structures[corrected]. Opt Lett. 2003. 28:2019–2021.

3. Hadjur C, Daty G, Madry G, Corcuff P. Cosmetic assessment of the human hair by confocal microscopy. Scanning. 2002. 24:59–64.

4. Baltenneck F, Bernard , Garson JC, Engström P, Riekel C, Leroy F, et al. Study of the keratinization process in human hair follicle by X-ray microdiffraction. Cell Mol Biol (Noisy-le-grand). 2000. 46:1017–1024.
5. James V, Corino G, Robertson T, Dutton N, Halas D, Boyd A, et al. Early diagnosis of breast cancer by hair diffraction. Int J Cancer. 2005. 114:969–972.

6. Rogers KD, Hall CJ, Hufton A, Wess TJ, Pinder SE, Siu K. Reproducibility of cancer diagnosis using hair. Int J Cancer. 2006. 118:1060.

7. Jung H, Kim HJ, Kim EK, Hong JO, Je JH, Hwu Y, et al. Comparison of unmonochromatized synchrotron radiation and conventional X-rays in the imaging of mammographic phantom and human breast specimens: a preliminary result. Yonsei Med J. 2005. 46:95–103.

8. Hwu Y, Tsai WL, Je JH, Seol SK, Kim B, Groso A, et al. Synchrotron microangiography with no contrast agent. Phys Med Biol. 2004. 49:501–508.

9. Neuhäusler U, Schneider G, Ludwig W, Meyer MA, Zschech E, Hambach D. X-ray microscopy in Zernike phase contrast mode at 4keV photon energy with 60 nm resolution. J Phys D Appl Phys. 2003. 36:A79–A82.
10. Youn HS, Baik SY, Chang CH. Hard x-ray microscopy with a 130 nm spatial resolution. Rev Sci Instrum. 2005. 76:023702. 1-4.
11. Youn HS, Jung SW. Observations of human hair shaft with an x-ray microscope. Phys Med Biol. 2005. 50:5417–5420.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download