Abstract
Purpose
Gastric cancer has the highest incidence rate among cancers in Asia. The advanced type of signet ring cell carcinoma has poor prognosis compared to other types of gastric cancer. The immuno-gene therapy with cytokine-based tumor vaccines has not yet been investigated for gastric cancer. The granulocyte macrophage colony-stimulating factor (GM-CSF)-based tumor vaccine has been demonstrated as the most potent stimulator for specific and long-lasting systemic tumor immunity.
Materials and Methods
In the present study, KATO III cells, the human signet ring cell gastric carcinoma cell line, were genetically modified by the transduction with the human GM-CSF cDNA or the modified hGM-CSF in replication-deficient retroviruses. The genomic integrations and mRNA expressions of the transgenes were determined by Southern and Northern blot analyses.
Results
Wild type (wt) or modified hGM-CSF was integrated into the genome of KATO III cells. The modified hGM-CSF mRNA was more stable than that of wt. The KATO III cells with the modified hGM-CSF produced higher level of hGM-CSF (12.4 - 19 ng/106 cells/48 hrs) than that with wt hGM-CSF, when determined by enzyme-linked immunosorbent assay (ELISA). The secreted recombinant hGM-CSF could support the proliferation of the GM-CSF-dependent cell line, indicating that the hGM-CSF secreted by the transduced KATO III cells has biological activities. Irradiated, transduced KATO III cells continued to secret hGM-CSF without proliferation.
Gastric cancer has the highest incidence rate in East Asia, including Korea and Japan.1 There are strong correlations between the high incidence rate of gastric cancer and the environment, especially childhood environment, diet, smoking, and Helicobacter pylori infection.2 Although the incidence rate of gastric cancer has been declined, it is the second cause of cancer-related death worldwide and the fourth common cancer.2
Surgery, chemotherapy and radiotherapy are generally applied for cancer therapy. However, the treatment with surgery alone has high rate of failure, and chemotherapy and radiotherapy have not improved the survival rates when used separately. Cancer vaccination is an active immunotherapy to induce active anti-tumor immunity of cancer patients by exposing the tumor antigens to their immune system. Cytokine-modified cell-based tumor vaccines have been clinically tested as immuno-gene therapy and major forms of cancer vaccines. Among genetically modified cell based-tumor vaccines to secrete cytokines, GM-CSF has been the most potent stimulator for specific and long-lasting systemic tumor immunity.3 GM-CSF is a critical factor for inducing differentiation of primitive hematopoietic precursors into dendritic cell (DC), the most potent professional antigen-presenting cells (APC) that initiates CD4+ and CD8+ T cell response.4-6 Paracrine GM-CSF from tumor cell vaccines may induce anti-tumor immune response by promoting local DC differentiation at vaccine site to induce local inflammatory response to eliminate tumors.
Autologous GM-CSF secreting tumor vaccines have cured the established tumors in mice and shown promising results in patients with melanoma and prostate and renal cell carcinomas.3,7-9 Since autologous GM-CSF secreting tumor vaccine cells display all the relevant tumor antigens, immunization with these cells will induce anti-tumor immunity to patients. Nevertheless, adequate numbers of these cells are rarely available and are labor intensive, because de novo gene transfer of each patient and certification of each patient's lot of vaccine cells are expensive and time-consuming.
To circumvent the limitations of autologous tumor vaccines, allogeneic tumor vaccines have been developed and reported to have numerous advantages over autologous cell counterparts. The rationale for allogeneic tumor vaccine includes the following. First, many tumor antigens seem to be commonly expressed among different tumors.10,11 Second, professional APCs are responsible for priming CD4+ and CD8+ T cells to generate systemic anti-tumor immunity.12,13 It has been demonstrated that tumor-specific CD8+ T cells are activated by cross-priming mechanism. Third, the efficacy of allogeneic tumor vaccine has been validated in animal models.7 Also, the irradiated allogeneic GM-CSF transduced tumor cell vaccine has been shown to be safe and induce dose-dependent systemic anti-tumor immunity in clinical trial of pancreatic adenocarcinoma, prostate cancer, lung cancer, leukemia and myeloma, as measured by increased post-vaccination delayed type hypersensitivity (DTH) response against the autologous tumor cells,3,14 indicating that immunizing tumor cells do not need to be HLA compatible with the host to generate tumor antigen-specific immunity. The trade-marked GM-CSF-based vaccines, GVAX™ series of cancer vaccines, have been successful in early clinical trials.15 Prostate cancer vaccine has been progressed to the phase III clinical trials while the pancreatic cancer, lung cancer, leukemia and myeloma vaccines have obtained positive phase II results.15
Immuno-gene therapies with cytokine-based tumor vaccines to treat gastric cancer have not so far been tested, even though many researchers have tried the cytokine-modified cancer cell immunotherapies for various types of cancers and observed positive pre-clinical and clinical results with GM-CSF-modified cancer vaccines.5,12,15 In this study, we established human gastric cancer cell lines that stably produced hGM-CSFs with replication-deficient retroviruses containing wt hGM-CSF cDNA or modified hGM-CSF. The modified hGM-CSF transgene transduced KATO III cells secreted higher levels of hGM-CSF than wt hGM-CSF transgene transduced cells. Also, this recombinant hGM-CSF had biological activity when tested with GM-CSF dependent cell growth analysis. These results indicate that the GM-CSF secreting KATO III cells can be tested as a potential GM-CSF secreting allogeneic tumor vaccine to treat gastric cancer as a part of an immunotherapeutic treatment.
Retroviral vectors, pLNCX and pLXIN (Clontech, Palo Alto, CA, USA), were used. Human GM-CSF cDNA was obtained from American Tissue Culture Collecton (ATCC No. 39754). KATO III cells, the human gastric cancer cell line (Korean Cell Line Bank No 30103, Seoul, Korea),16 and PA317 packaging cell lines were maintained in RPMI 1640 medium and Dulbecco's modified Eagle's medium, respectively, supplemented with 10% heat inactivated fetal bovine serum, 100 IU/mL penicillin and 100 µg/mL streptomycin, and grown at 37℃ with 5% CO2.
The hGM-CSF cDNA (p91023, ATCC No. 39745) was cloned into Hpa I site of pLNCX retroviral vector, and Hpa I and Xho I sites of pLXIN retroviral vector, resulting in pLNCGM and pLGMIN, respectively. Green fluorescence protein (GFP) gene was cloned into Hpa I and BamH I sites of retroviral vectors, pLNCX and pLXIN, respectively. To modify the hGM-CSF gene, hGM-CSF AUG start codon flanking sequences were replaced to a partial Kozak sequences, and 3'-end UTR of AUUUA repeat sequences of the hGMCSF gene was replaced to AUGUA sequences from nt 672 to 688, and deleted from nts 647 to 671 and from nts 689 to 711 sequences using a recombinant polymerase chain reaction (PCR).17 In brief, to modify the AUG start codon flanking sequences and 3'-end UTR of hGM-CSF cDNA, hGM-CSF cDNA in pcDNA3 vector was incubated with sense primer GC6 (5'-CGGGATCCGCCACCATGTGGCTGCAGAGCCTGCTGCTC-3'), which contains Kozak sequence and BamH I site and binds nt 9 to 32 of hGM-CSF cDNA, and anti-sense primer GC10 (5'-TACATACATACATACATA TTACTGATTTCTGTCAGTATAA-3'), which binds nt 626 to 686 with the deletion from 647 to 671 and the modification from nt 672 to 688, in PCR buffer at 94℃ for 1 min, 54℃ for 1 min, and 72℃ for 1 min for 25 cycles. Also, hGM-CSF cDNA in pcDNA3 vector was incubated with sense primer GC11 (5'-ATGTATGTATGTATGTATTCAAGATG TTTTACCGT-3'), which binds to nts 669 to 733 with the deletion from 689 to 711 and the modification from nt 672 to 688, and anti-sense primer GC9 (5'-CGGGATCCGTGTGATGGATAT CTGCAGAATTC-3'), which binds pcDNA vector sequences with BamH I site, as described above. The resulting 680 bp and 120 bp PCR fragments were purified and combined for recombinant PCR using 94℃ for 1 min, 42℃ for 1 min, and 72℃ for 1 min for 25 cycles, with primers GC6 and GC9. The 750 bp amplified PCR product was purified and cloned into Hpa I and BamH I sites of pLNCX and pLXIN retroviral vectors, resulting in pLNCGMH and pLGMHIN, respectively. The entire PCR derived regions were sequenced to confirm the presence of the specific mutations and the absence of other mutations.
Wt and modified hGM-CSFs and GFP genes in pLNCX and pLXIN vectors were transfected to PA317 packaging cell line by lipofectamine reagent (Invitrogen™). The hGM-CSF containing retrovirus particles were harvested and then infected to KATO III cells in the presence of 8 µg/mL of polybrene. The colonies of transduced KATO III cell were selected in media containing 0.5 mg/mL of G418 and cultured. RT-PCRs with total RNA extracts were performed using hGM-CSF specific primers to detect the expression of hGM-CSF mRNA.
The concentration of hGM-CSF secreted from transduced KATO III cells was determined by hGM-CSF ELISA kit (Endogen, Woburn, MA, USA).
Chromosomal DNA was extracted by Easy-DNA™ (Invitrogen™) according to manufacturer's instruction. Thirty µg of chromosomal DNA was digested with Xho I, resolved in 0.8% agarose gels and transferred to nylon membrane. The DNA on the membrane was hybridized with 32P-labeled random-primed probe specific for GM-CSF sequences. To analyze hGM-CSF mRNA, total RNA was extracted by RNA stat 60 (Tel-test, Inc., USA) according to manufacturer's instruction. Twenty-five µg of total RNA was denatured and electrophoresed through 1% agarose gel containing formaldehyde, and blotted onto nylon membrane. The GM-CSF mRNA on the membrane was hybridized with 32P-labeled random-primed probe specific for GM-CSF.
The transduced KATO III cells (3 × 106 cells) were irradiated twice with 30 Gray of γ-ray and then incubated at 37℃ with 5% CO2. Cell proliferation was monitored by cell count every 2 days.
AML-193 (acute myeloid leukemia cell), GM-CSF dependent leukemic cell line,18 was used to test the biological activity of recombinant hGM-CSF. In brief, AML-193 cells were cultured in the presence of transduced KATO III cell culture supernatant, which contains the recombinant hGM-CSF secreted. AML-193 cell proliferation was monitored by 3H-thymidine uptake.
Replication-defective retroviral vector, pLXIN (Clontech), that expresses foreign gene under the control of long terminal repeat (LTR) promoter with additional attenuated internal ribosome entry site (IRES) and neomycin-resistance gene to ensure the expression of foreign gene after G418 selections, was used to construct the pLGMIN that contains cloned wt hGM-CSF cDNA. The retroviruses with wt hGM-CSF cDNA from the pLGMIN transfected-PA317 packaging cells were infected into KATO III cells to select the hGM-CSF cDNA transduced KATO III cells. However, the hGM-CSF (452 - 600 pg/106 cells/48 hrs) secreted from transduced KATO III cell with LGMIN vector was too low for therapeutic doses (Table 1).
To increase the expression of hGM-CSF, we modified the AU rich element in 3'-end UTR of the hGM-CSF gene (Fig. 1) to lengthen the half-life of hGM-CSF mRNA in vitro by blocking the post-transcriptional degradation.17 In addition to the modified 3'-end UTR, we also modified the 5'-end AUG start codon flanking sequence to a partial Kozak sequences (Fig. 1) to up-regulate the translation efficiency of hGM-CSF mRNA.19 Thus, KATO III cells were transduced with LGMHIN, the retroviruses with modified hGM-CSF cDNA, as described above. The hGM-CSF secreted from modified hGM-CSF transduced KATO III cells was 12 - 19 ng/106/48 hrs (Table 1). Since it has been suggested that the amount of secreted hGM-CSF for therapeutic uses needs to be moderate but not too high or low,20 the hGM-CSF secreted from the presently modified hGM-CSF transduced KATO III cells was in the almost right range although a little low. Therefore, the established hGM-CSF secreting transduced KATO III cells in this study could be tested for gastric cancer immunotherapy.
To test whether hGM-CSF was integrated into the chromosome of transduced KATO III cells, genomic DNAs of two representative KATO III cell clones, containing LGMIN or LGMHIN, were analyzed by Southern blot analysis (Fig. 2). Due to their random integration sites and single Xba I site in pLXIN vector, when digested with Xba I, a single band of various sizes would indicate that hGM-CSF was integrated as a single copy. As expected, each clone of transduced KATO III cells showed a single band of the hGM-CSF gene that was different between two clones (Fig. 2). After several passages, hGM-CSF transduced KATO III cells were found to constantly secrete the amount of hGM-CSF similar to that seen in Table 1 (data not shown), indicating that hGM-CSF transgenes containing KATO III cells could stably express hGM-CSF through integrated hGM-CSF.
Next, we examined mRNA expressions of the wt and modified hGM-CSFs by Northern blot analysis (Fig. 3). Since the cloned wt and modified hGM-CSFs cDNAs in pLXIN vector have their own polyadenylation site, wt and modified hGM-CSF mRNAs could be expressed as two mRNA species. One is the authentic wt or modified hGM-CSF mRNAs and the other is bicistronic mRNAs which contain the wt or modified hGM-CSF and IRES sequences and the neomycin resistance gene. With the hGM-CSF specific probe, we detected two mRNA species which were approximately 3.6 and 0.9 kbs in length (data not shown). The expressions of 3.6 and 0.9 kbs hGM-CSF mRNAs appeared to be not significantly different between the wt and modified hGM-CSF mRNAs (Fig. 3, data not shown), suggesting that hGM-CSF mRNA was actively synthesized in the wt and modified hGM-CSF transduced KATO III cells.
Because of no noticeable differences in the expression levels between the wt and modified hGM-CSF mRNAs (Fig. 3), we next compared the stability of the wt and modified hGM-CSF mRNAs by treating the transduced KATO III cells with 2.5 mg/mL of actinomycin-D for 3 hrs to block new mRNA synthesis (Fig. 4), and the intensities of modified hGM-CSF mRNA were stronger than those of wt hGM-CSF mRNAs by the treatment (Fig. 4), indicating that modified hGM-CSF mRNAs were stable and not degraded easily like wt mRNA.17 Therefore, the modified hGM-CSF-transgene transduced KATO III cells would appear to secrete more hGM-CSFs and/or longer period of time. As expected, the modified hGM-CSF transduced KATO III cells continued to secrete hGM-CSF in the presence of actinomycin-D, indicating that the already existing hGM-CSF mRNA continues to be translated to secrete hGM-CSF (data not shown).
Since we modified only the 5'- and 3'-ends of UTR of hGM-CSF cDNA (Fig. 1), the hGM-CSF secreted from the wt and modified hGM-CSF should have same biological activities. To test the above contention, the proliferation of AML-193 cell, the GM-CSF dependent leukemic cell line, was monitored by 3H-thymidine uptake (Fig. 5).18 Thus, the culture supernatant from the clone number 18 of transduced KATO III cells, which secreted 18 - 20 ng/106 cells/48 hrs of hGM-CSF (Table 1), was applied to AML-193 cells. As shown in Fig. 5, AML-193 cells were found to proliferate by the addition of the culture supernatant, confirming biological activities of the recombinant hGM-CSF.
Since we showed that the modified hGM-CSF mRNA is more stable than the wt hGM-CSF mRNA (Fig. 4), the modified hGM-CSF transduced KATO III cells were irradiated to test whether the irradiated KATO III cells with transgenes continue to secrete hGM-CSF without cell proliferation. After twice the irradiation by 30 Gray of γ-ray, the KATO III cells no longer proliferated for 8 days (data not shown), indicating that irradiation by 30 Gray of γ-ray twice is sufficient to stop proliferation of KATO III cells. Recombinant hGM-CSF was continuously secreted from the irradiated, transduced KATO III cells (Table 2), suggesting that these hGM-CSF secreting KATO III cells could be tested as a potential allogeneic gastric tumor vaccine to treat gastric cancer.
To treat cancers, several strategies of gene therapies, including induction of malignant cell death, modulation of immune response to tumors, and reversion of malignant process by correcting the genetic abnormalities, have been applied. In addition, gene replacement, anti-sense therapy, cytotoxic gene therapy, immune-gene therapy, and drug resistance transfer have also been applied. Immunotherapy to modulate immune response to a particular tumor can be divided into passive and active immunotherapies. Passive immunotherapy involves the transfer of immune effectors such as tumor-specific T cells and tumor-specific antibodies. Immunizations with killed tumor cells or purified tumor antigens, or with tumor antigen-transfected or -pulsed professional antigen presenting cells (APCs) are parts of active immunotherapies. Cytokine- or costimulatory-, such as the B7 gene, modified cancer cell vaccines also constitutes active immunotherapies.
It had been reported that the advanced type of signet ring cell carcinoma has poor prognosis compared to other types of gastric cancer.21 Therefore, alternative or supplementary treatments to augment chemotherapy or surgery, such as immunotherapy, are in great demand. KATO III cells, the signet ring cell gastric carcinoma cell line, are diffuse type undifferenciated adenocarcinoma cells with the deletion of p53, the amplification of K-sam and c-met oncogenes, and the mutations of E-cadherin1,16,22,23 and express HLA-ABC, HLA-A2/A28, HLA-B7/B27, HLA-DR and HLA-DQ.24 Since KATO III cells do not express GM-CSF mRNA when infected with H. pylori25 and do not secrete hGM-CSF, KATO III cells were selected in this study to genetically modify and secrete hGM-CSF, as a candidate of hGM-CSF-modified human gastric cancer cell vaccine.
Since retroviruses do not have lytic cycle and stably express the chromosome-integrated foreign gene in infected cells, retroviruses are the most commonly utilized vectors to prepare cytokine-secreting tumor vaccines. The fact that PA317 packaging cell line, used in this study, produced high titer of hGM-CSF cDNA containing retroviruses in the absence of helper virus with amphotropic host range indicates that various types of cells, including mouse, rat, cat, dog and human cells, such as hematopoietic progenitor cells from human bone marrow, could be infected.26 Among two pLNCX and pLXIN retroviral vectors tested to deliver the hGM-CSF gene into KATO III cells, we could not obtain wt hGM-CSF transduced KATO III cell clones using pLNCX vector even though we were able to detect small amount of hGM-CSF secreted from pooled KATO III cells with transgene (data not shown). Although modified hGM-CSF transduced KATO III cell clones were obtained using pLNCX vector and secreted amounts of hGM-CSFs were detectable, the amount of secreted hGM-CSF was still too low for GM-CSF secreting cancer vaccine trial (data not shown). We speculate that some hGM-CSF were lost from LNCGM and LNCGMH retroviruses from transduced, G418 selected-KATO III cells during the selection processes that select only by neomycin resistance. On the other hand, neomycin resistance gene in LGMIN and LGMHIN retroviruses was expressed under the control of 5'-end LTR promoter through attenuated IRES from the same transcript to ensure the expression of wt and modified hGM-CSF after G418 selection.
The expressions of most cytokines or growth factors are modulated by self-regulatory mechanism by controlling mRNA level. Most cytokine mRNAs, including GM-CSF, contain adenosine-uridine (AU)-rich element (AREs) in the 3'-end untranslated region (UTR) which is the target site for RNA binding protein to control the expression level through the regulated mRNA stabilities.17,27 The AREs, pentameric AUUUA sequences, in 3'-UTR regions of cytokine genes are AUUUA instability elements that are targeted by RNA binding protein, adenosine-uridine binding factor (AUBF), to prevent rapid decay of mRNAs in activated cells.17,27-29 When AUBF activity is depleted, GM-CSF mRNA decay is accelerated from 90 min half-life to 20 min, indicating that mRNA stability is regulated through changes in half-lives.28,29 The mutations of this ARE increase the GM-CSF mRNA stability by 5-fold, thus elevating the expression of GM-CSF without affecting the biological activities.16,17 Also, due to the suboptimal start codon, cytokine mRNAs are poor initiators of translation to modulate the yield of cytokine.19 In the present study, since hGM-CSF secretion by wt hGM-CSF transduced KATO III was too low to modulate immune responses, we transduced KATO III cells with modified transgene in which both the 5'-end suboptimal start codon and 3'-end AUUUA instability elements of hGM-CSF were mutated to increase the steady state level, resulting in 20- to 40-fold increases of hGM-CSF secretion (Table 1).
It had been demonstrated that vaccines producing ≥ 40 ng of GM-CSF/106 cells/24 hrs induced maximal systemic immunity.20 The modified hGM-CSF transduced KATO III cells produced 12 - 19 ng/106 cells/48 hrs (Table 1), which is slightly low for the maximal systemic immunity, nevertheless, still in the range to be effective.20 Therefore, we suggest that it would be worthwhile to test the irradiated, hGM-CSF secreting KATO III cells as an allogeneic tumor vaccine to treat gastric cancer as a part of immunotherapeutic treatment.
Figures and Tables
 | Fig. 1Targeted mutagenesis of human GM-CSF sequence. To increase the hGM-CSF expressions, both the 5'-end start codon flanking sequences (A) and 3'-end UTR (B) were mutated using recombinant PCR techniques. (A) The suboptimal AUG start codon flanking sequences were replaced to a part of Kozak sequences. (B) AUUUA repeat sequences in 3'-end UTR of the hGM-CSF gene were mutated to AUGUA repeat sequences (nts 672-688) and deleted (nts 647-671 and 689-711). |
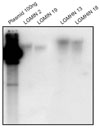 | Fig. 2Chromosomal integrations of the wt and modified hGM-CSF transgenes in the transduced KATO III cells. Southern blot analysis was performed to detect the integrated wt and modified hGM-CSF transgenes in the respective transduced KATO III cells. Chromosomal DNA was isolated from two representative clones of LGMIN- and LGMHIN-transduced KATO-III cells. Thirty µg of Xba I-digested chromosomal DNA was resolved in 0.8% agarose gels and transferred to nylon membrane. The DNA on the membrane was hybridized with random-primed 32P-labeled GM-CSF specific probe and subjected to autoradiography. |
 | Fig. 3The hGM-CSF mRNA expressions in the wt and modified hGM-CSF transduced KATO III cells. Northern blot analysis was performed to detect the wt and modified hGM-CSF mRNAs in the respective transduced KATO III cells. Total RNA was isolated from four representative clones of LGMIN- and LGMHIN-transduced KATO III cells. Transduced KATO III cells with LGFPIN, GFP-containing retrovirus, were used as a negative control. Twenty-five µg of total RNA was denatured and electrophoresed through 1% agarose gel containing formaldehyde and transferred to nylon membrane. The GM-CSF mRNA on the membrane was hybridized with random-primed 32P-labeled GM-CSF specific probe and subjected to autoradiography. 18S ribosomal RNAs are indicated as a loading control. |
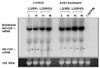 | Fig. 4The stabilities of wt and modified hGM-CSF mRNAs in the wt and modified hGM-CSF transduced KATO III cells. Northern blot analysis was performed to compare the mRNA stabilities of wt and modified hGM-CSF mRNAs. LGFPIN transduced KATO III cells were used as a negative control. Two representative clones of LGMIN and LGMHIN transduced KATO III cells were treated with 2.5 mg/mL of actinomycin-D for 3 hrs, and total RNA was isolated. Twenty-five µg of total RNA was denatured and electrophoresed through 1% agarose gel containing formaldehyde, and blotted onto nylon membrane. GM-CSF mRNA on the membrane was hybridized with random-primed 32P-labeled GM-CSF-specific probe and subjected to autoradiography. 0.9kbs GM-CSF mRNAs from LGMIN and LGMHIN transduced KATO III cells are indicated as squares and asterisks, respectively. 18S ribosomal RNAs are indicated as a loading control. |
 | Fig. 5Proliferation of GM-CSF dependent leukemic cells by the addition of recombinant hGM-CSF from LGMHIN transduced KATO III cells. To examine biological activity of the recombinant hGM-CSF, culture supernatant from a clone 18 of LGMHIN-transduced KATO III cells was added to AML-193 cells (GM-CSF dependent leukemic cell line) and proliferation of AML-193 cells was monitored by 3H-thymidine uptake. |
Table 2
hGM-CSF Secretion from the Irradiated, hGM-CSF-Transduced KATO III Cells

Two representative clones of LGMIN- and LGMHIN-transduced KATO-III cells were selected. Each set of 3 × 106 cells were irradiated two times with 30 Gray of γ-ray, then incubated at 37℃ with 5% CO2 for 2 days. ELISA was performed to detect hGM-CSF from cell culture supernatant using human GM-CSF ELISA kit (Endogen, Woburn, MA, USA). Concentration was ng/106 cells/48 hrs.
References
1. Yokozaki H. Molecular characteristics of eight gastric cancer cell lines established in Japan. Pathol Int. 2000. 50:767–777.

2. Konturek PC, Konturek SJ, Brzozowski T. Gastric cancer and Helicobacter pylori infection. J Physiol Pharmacol. 2006. 57:Suppl 3. 51–65.
3. Dranoff G, Jaffee E, Lazenby A, Golumbek P, Levitsky H, Brose K, et al. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci U S A. 1993. 90:3539–3543.

4. Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998. 392:245–252.

5. Inaba K, Steinman RM, Pack MW, Aya H, Inaba M, Sudo T, et al. Identification of proliferating dendritic cell precursors in mouse blood. J Exp Med. 1992. 175:1157–1167.

6. Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, et al. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992. 176:1693–1702.

8. Soiffer R, Lynch T, Mihm M, Jung K, Rhuda C, Schmollinger JC, et al. Vaccination with irradiated autologous melanoma cells engineered to secrete human granulocyte-macrophage colony-stimulating factor generates potent antitumor immunity in patients with metastatic melanoma. Proc Natl Acad Sci U S A. 1998. 95:13141–13146.

9. Simons JW, Mikhak B, Chang JF, DeMarzo AM, Carducci MA, Lim M, et al. Induction of immunity to prostate cancer antigens: results of a clinical trial of vaccination with irradiated autologous prostate tumor cells engineered to secrete granulocyte-macrophage colony-stimulating factor using ex vivo gene transfer. Cancer Res. 1999. 59:5160–5168.
10. Rosenberg SA, Kawakami Y, Robbins PF, Wang R. Identification of the genes encoding cancer antigens: implications for cancer immunotherapy. Adv Cancer Res. 1996. 70:145–177.

11. Boon T, van der Bruggen P. Human tumor antigens recognized by T lymphocytes. J Exp Med. 1996. 183:725–729.

12. Huang AY, Bruce AT, Pardoll DM, Levitsky HI. In vivo cross-priming of MHC class I-restricted antigens requires the TAP transporter. Immunity. 1996. 4:349–355.

13. Huang AY, Golumbek P, Ahmadzadeh M, Jaffee E, Pardoll D, Levitsky H. Role of bone marrow-derived cells in presenting MHC class I-restricted tumor antigens. Science. 1994. 264:961–965.

14. Jaffee EM, Hruban RH, Biedrzycki B, Laheru D, Schepers K, Sauter PR, et al. Novel allogeneic granulocyte-macrophage colony-stimulating factor-secreting tumor vaccine for pancreatic cancer: a phase I trial of safety and immune activation. J Clin Oncol. 2001. 19:145–156.

15. Hege KM, Jooss K, Pardoll D. GM-CSF gene-modified cancer cell immunotherapies: of mice and men. Int Rev Immunol. 2006. 25:321–352.
16. Sekiguchi M, Sakakibara K, Fujii G. Establishment of cultured cell lines derived from a human gastric carcinoma. Jpn J Exp Med. 1978. 48:61–68.
17. Rajagopalan LE, Burkholder JK, Turner J, Culp J, Yang NS, Malter JS. Granulocyte-macrophage colony- stimulating factor mRNA stabilization enhances transgenic expression in normal cells and tissues. Blood. 1995. 86:2551–2558.

18. Lange B, Valtieri M, Santoli D, Caracciolo D, Mavilio F, Gemperlein I, et al. Growth factor requirements of childhood acute leukemia: establishment of GM-CSF-dependent cell lines. Blood. 1987. 70:192–199.

19. Kozak M. An analysis of vertebrate mRNA sequences: intimations of translational control. J Cell Biol. 1991. 115:887–903.

20. Jaffee EM, Pardoll DM. Considerations for the clinical development of cytokine gene-transduced tumor cell vaccines. Methods. 1997. 12:143–153.

21. Yokota T, Kunii Y, Teshima S, Yamada Y, Saito T, Kikuchi S, et al. Signet ring cell carcinoma of the stomach: a clinicopathological comparison with the other histological types. Tohoku J Exp Med. 1998. 186:121–130.

22. Oda T, Kanai Y, Oyama T, Yoshiura K, Shimoyama Y, Birchmeier W, et al. E-cadherin gene mutations in human gastric carcinoma cell lines. Proc Natl Acad Sci U S A. 1994. 91:1858–1862.
23. Matozaki T, Sakamoto C, Matsuda K, Suzuki T, Konda Y, Nakano O, et al. Missense mutations and a deletion of the p53 gene in human gastric cancer. Biochem Biophys Res Commun. 1992. 182:215–223.

24. Iguchi C, Nio Y, Takeda H, Yamasawa K, Hirahara N, Toga T, et al. Plant polysaccharide PSK: cytostatic effects on growth and invasion; modulating effect on the expression of HLA and adhesion molecules on human gastric and colonic tumor cell surface. Anticancer Res. 2001. 21:1007–1013.
25. Jung HC, Kim JM, Song IS, Kim CY. Helicobacter pylori induces an array of pro-inflammatory cytokines in human gastric epithelial cells: quantification of mRNA for interleukin-8, -1 alpha/beta, granulocyte-macrophage colony-stimulating factor, monocyte chemoattractant protein-1 and tumour necrosis factor-alpha. J Gastroenterol Hepatol. 1997. 12:473–480.

26. Miller AD, Buttimore C. Redesign of retrovirus packaging cell lines to avoid recombination leading to helper virus production. Mol Cell Biol. 1986. 6:2895–2902.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download