Abstract
Purpose
The precise molecular mechanisms culminating in coronary artery disease (CAD) are not well understood, despite a wealth of knowledge on predisposing risk factors and pathomechanisms. CAD and myocardial infarction (MI) are complex genetic diseases; neither the environment alone, nor a single gene, cause disease, rather, a mix of environmental and genetic factors lead to atherosclerosis of the coronary arteries.
Materials and Methods
In the present study, our aim was to investigate the roles of prothrombin G20210A mutation and Factor VLeiden mutation in atherosclerotic coronary artery disease. 287 subjects (106 control subjects, who were angiographically normal, and 181 angiographically documented coronary atherosclerotic patients who exhibited coronary artery narrowing to a degree of ≥ 50%) were included in this study. The mutations were assessed with LightCycler Real-Time PCR mutation detection kits (Roche Diagnostics, GmbH, Germany).
Results
6.6% of control subjects, and 6.1% of patients with (50% coronary artery narrowing were determined to have the Factor VLeiden heterozygote mutation. 6.6% of control subjects had the Prothrombin G20210A heterozygote mutation, while 7.7% of patients with (50% coronary artery narrowing had this mutation. The OR for Factor VLeiden was 1.52 (CI: 0.240 - 9.602) and for Prothrombin G20210A mutation, the OR was 1.415 (CI: 0.287 - 6.962).
Cardiovascular disease is the result of an interaction between environmental influences and genetic predisposition. In addition to the well-accepted traditional risk factors, there is increasing evidence suggesting that coagulation may be involved in the pathogenesis of atherosclerosis, and also in the clinical progression to plaque rupture and localized occlusive thrombus formation.1
The association between environmental factors and myocardial infarction has been thoroughly investigated, but the role of genetic markers is still poorly defined.2 Over the past few years, studies have focused on the role of haemostatic markers, which reflect inherited or acquired propensities for thrombosis and/or the extent of subclinical atherosclerosis, and several genetic mutations affecting coagulation proteins, which have been suggested as prothrombotic risk factors.3,4
Several studies have recently examined the relationship between myocardial infarction and prothrombotic genetic markers, such as the 4G/5G polymorphism of the PAI-1 gene promoter, PIA1/PIA2 polymorphism of the platelet glycoprotein IIIa gene, C3550T polymorphism of the platelet glycoprotein Ib gene, C677T polymorphism of the methylenetetrahydrofolate reductase gene, and the G10976A polymorphism (Arg353Gln) of the factor VII gene. However, conclusions regarding genotype and allele frequencies have been inconsistent. 5-7 Two other polymorphisms, the G1691A mutation in the Factor V gene,8 and the G20210A mutation in the prothrombin gene,9 are definitely associated with an increased risk of venous thrombosis, but whether they are associated with a risk of arterial thrombosis remains controversial.
The Prothrombin G20210A mutation was associated with a higher prothrombin clotting activity and a 2- to 7- fold increase in risk of venous thrombosis.9 Other groups reported similar findings.10-12 The role of the prothrombin gene G20210A variant in CAD has not yet been established. Several investigators, however, have reported a significant increase in the prevalence of the prothrombin gene G20210A variant in patients with CAD (1.8% to 12.5%), compared with newborns or age-matched controls,13-15 and a 4-fold increase in risk of myocardial infarction in young women with this variant.16 Others found no increased prevalence of the prothrombin gene G20210A variant in patients with CAD, compared with age and sex-matched controls.17-19
The relationship between the Factor VLeiden mutation and CAD is also still controversial. Several investigators have uncovered a significant association between Factor VLeiden and coronary artery disease,20-22 or have found an increased prevalence of Active Protein C (APC) resistance in stroke patients,23 whereas other groups reported no association between APC resistance or Factor VLeiden and coronary artery disease23-25 or ischemic stroke15,18,24 respectively.
In the present study, we investigated a possible association between Factor V, Prothrombin G20210A mutation and subjects with angiographically documented CAD.
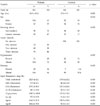
287 subjects (106 controls, who were angiographically normal, and 181 angiographically documented coronary atherosclerotic patients who had (50% coronary artery narrowing) were included in this study. Of one hundred and eighty-one patients, 114 males (58.2 ± 10) and 67 females (58.5 ± 10) underwent percutaneous transluminal coronary angioplasty (PTCA) or coronary bypass grafting (CABG) surgery, with diagnoses of 50% stenosis in at least one of the major coronary arteries constituting the study group. The other 52 male (57.6 ± 10) and 54 female (57.1 ± 10) subjects, whose coronary angiographies turned out to be normal, formed the control group. The patients who had triglyceride levels of more than 400 mg/dL were excluded because Low Density Lipoprotein (LDL) content is calculated from primary measurements using the empirical equation. The demographic data of patients and controls are given in Table 1. The patients and controls were from the same geographic region and of the same ethnic origin. Also, cases and controls were unrelated. This study was carried out according to the principles of the Declaration of Helsinki, and was approved by the Mersin University School of Medicine, and an investigational review board. Informed consent was obtained from all participating patients.
Venous blood was collected by venipuncture, in sterile siliconized EDTA 2-mL Vacutainer tubes, and stored at + 4℃ until time of analysis. Genomic DNA was extracted from whole blood using High Pure PCR Template Preparation kits (Roche Diagnostics, GmbH, Germany).
To determine what, if any, mutations were harboured in the samples, we used the LightCycler Factor VLeiden Mutation Detection Kit (catalog number: 2 212 161) and the LightCycler Prothrombin G20210A Mutation Detection Kit (catalog number: 2 236 842) (Roche Diagnostics, GmbH, Mannheim, Germany).
A fragment (222 bp for Factor V, 165 bp for Prothrombin gene) of the relevant gene was amplified using specific primers from human genomic DNA. The amplicon was detected by fluorescence using a pair of hybridization probes. The hybridization probes were also used to determine the genotype, by performing a melting curve analysis, after completion of the amplification cycles.
In this analysis, the differences between the mutant and wild type of the related genes were determined by assessing differences in their melting temperatures. The mutant amplicon had a point mutation which mismatched the hybridization probes, and so left them earlier than did the wild type.
Apolipoprotein A (Apo A), Apolipoprotein B (Apo B) and lipoprotein (a) (Lp a) were determined by immunoturbidometric methods. Triglycerides (TG), Total cholesterol (TC) and High Density Lipoprotein (HDL) were analyzed by GPO/PAP enzymatic colorimetric, CHOD/PAP enzymatic colorimetric, and direct COHD/PAP enzymatic colorimetric methods, respectively. Low Density Lipoprotein (LDL) content was calculated from the primary measurements using the empirical equation. All these parameters were determined using a Cobas Integra 700 (Hitachi Modular Systems) biochemical analyzer (Roche Diagnostics, GmbH, Mannheim, Germany).
We used percentages to express the distribution of the mutations in the control and CAD groups. Risk estimates were accomplished by logistic regression analysis, in order to ameliorate the effects of known risk factors such as hypertension, diabetes mellitus, cigarette smoking, and lipid parameters, on Factor VLeiden and Prothrombin G20210A mutations. Risks are defined as the odds ratio (OR) coupled with a confidence interval (CI). All these statistical analyses were made using SPSS 9.05 for Windows Version statistics program and Minitab statistical software (Minitab release 13.0).
We investigated the distribution of Factor VLeiden and Prothrombin G20210A mutation among patients with (50% coronary artery narrowing, and control subjects who were angiographically normal (Tables 2 and 3). Seven of 106 control subjects, and 11 of 181 patients with (50 coronary artery narrowing were found to have the Factor VLeiden mutation (Table 2). With regard to Prothrombin G20210A, there were 7 heterozygote genotype control subjects and 14 heterozygote genotypes, in patients with (50% coronary artery narrowing (Table 3). The OR for Factor VLeiden was 1.52 (CI: 0.240 - 9.602), and for Prothrombin G20210A mutation, the OR was 1.415 (CI: 0.287 - 6.962). Homozygotic mutations of Factor VLeiden and Prothrombin G20210A were not detected in either the control or the patient groups. The mean and standard deviation of total cholesterol, HDL-cholesterol, LDL-cholesterol, VLDL-cholesterol, triglycerides, Lipoprotein (a), Apolipoprotein A and Apolipoprotein B of control and patients can be seen in Table 1.
Coronary artery disease (CAD) is a significant cause of morbidity and mortality today. Although CAD treatment protocols are improving, its prevalence has increased. Both primary and secondary prevention measures are of vital importance.26 Atherosclerosis is the principal cause of the cardiovascular diseases.27 Acute myocardial infarction (AMI) frequently results from the rupture of an atherosclerotic plaque, followed by thrombus formation.28 Major risk factors for atherosclerosis include: current smoking, hypertension, diabetes mellitus, and dyslipidemia, including the apolipoprotein E4 isoform.29
The contribution of thrombogenic risk factors to the development of AMI has been less well characterized. Potential risk factors that have been studied include: abnormalities of blood flow, platelelet hyperactivity, reduced fibrinolysis, and increased plasma levels of fibrinogen, factor VII, von Willebrand factor, tissue plasminogen activator, and tissue plasminogen activator inhibitor.30 Several genetic mutations affecting coagulation proteins have been suggested as prothrombotic risk factors.15 Among these, the Prothrombin gene and Factor VLeiden mutation have recently been studied as potential candidates. Therefore we attempted to determine whether the G20210A Prothrombin mutation and Factor VLeiden were associated with CAD in the population-based case control study.
Prothrombin is a precursor of the serine protease thrombin, a key enzyme acting as a procoagulant, via platelet activation and the generation of fibrin and Factors Va, VIIIa and XIIIa, and subsequently as an anticoagulant, by activating circulating protein C. Therefore, regulation of thrombin activity is crucial for the maintenance of haemostatic balance.31
In 1996, Poort et al.9 described a variant of the prothrombin gene that was associated with higher prothrombin levels, and with an increased risk for venous thrombosis. Several other studies later confirmed these initial observations. However, the potential role of this mutation in atherothrombotic disease remains highly controversial.31
Excessive thrombin generation has been described in individuals at high risk of fatal CAD. It seems biologically plausible that the higher prothrombin levels related to the G20210A variant may also confer an increased risk of CAD. To date, however, studies attempting to answer this question have yielded conflicting results. In some reports, being a carrier of the mutation was associated with an increased risk for myocardial infarction.16,32-34 Nevertheless, the only prospective study published so far failed to establish any association between the G20210A allele and MI.35
In our study, 6.6% of control subjects harbored the Prothrombin G20210A mutation, while 7.7% of patients with coronary artery narrowing had this mutation. There was found to be no difference in the distribution of the prothrombin G20210A mutation between patients and controls (Table 3).
Another possible prothrombotic risk factor is the Factor VLeiden mutation. Factor VLeiden is the most commonly known hereditary abnormality of the clotting system, with a 3% to 5% prevalence of heterozygote carriers. Due to mutations (Arg 506Gln or R506Q) at the cleavage site for activated protein C (APC), clotting Factor V is inactivated at a reduced rate. This defect leads to a reduced anticoagulant effect of APC, with a less-than-expected prolongation of the activated partial thromboplastin time (APTT). The reduced anticoagulant effect of the protein C/Protein S natural inhibitor system leads to an increased tendency to thrombosis.22
Several studies have demonstrated an association between resistance to activated protein C and venous thrombosis.36,37 Heterozygotes for this abnormality are commonly found among unselected patients with deep-vein thrombosis (20%), and are even more prevalent among referred patients or patients with familial thrombophilia (40 - 60%). Compared to those without the mutation, heterozygote carriers of the mutation carry an increased risk of venous thrombosis.22
Whether Factor VLeiden has any effect whatsoever on the risk for arterial disease is arguable, and few studies have investigated the association. Several reports, including a controlled study among patients with coronary stenosis, are suggestive of an association with coronary heart disease,16,22,32 but in several other controlled studies no relationship was observed.36-39
In our study, we determined there to be no statistical relation between the control group, and the (50% coronary artery narrowing patients, in terms of their Factor VLeiden mutation frequencies. 6.6% of control subjects and 6.1% of patients with (50% coronary artery narrowing have Factor VLeiden mutation. There was found to be no difference in the distribution of Factor VLeiden mutation between patients and controls (Table 2).
The two mutations considered to be risk factors for venous thrombosis are the Factor VLeiden mutation and the Prothrombin G20210A mutation. In our study, we investigated whether these mutations might be risk factors for coronary artery disease. Their roles in coronary artery disease were controversial. Although heterozygote Factor VLeiden and the Prothrombin gene mutation were more frequent in patients with CAD than in control subjects, there was found to be no statistical relationship between coronary artery disease and the Factor VLeiden and Prothrombin G20210A mutations.
Figures and Tables
References
1. Zaman AG, Helft G, Worthley SG, Badimon JJ. The role of plaque rupture and thrombosis in coronary artery disease. Atherosclerosis. 2000. 149:251–266.

2. Ardissino BY, Mannucci PM, Mertini PA, Duca F, Fetiveau R, Tagliabue L, Tubaro M, et al. Prothrombotic genetic risk factors in young survivors of myocardial infarction. Blood. 1999. 94:46–51.

3. Ridker PM. Fibrinolytic and inflammatory markers for arterial occlusion: the evolving epidemiology of thrombosis and hemostasis. Thromb Haemost. 1997. 78:53–59.

4. Cushman M. Schechter GP, Hoffman R, Schrier SL, editors. Hemostatic risk factors for cardiovascular disease. Hematology. 1999. Washington. DC: American Society of Haematology.
5. Di Minno G, Grandone E, Margaglione M. Clinical relevance of polymorphic markers of arterial thrombosis. Thromb Haemost. 1997. 78:462–466.

6. Grant PJ. Polymorphisms of coagulation/fibrinolysis genes: gene environment interactions and vascular risk. Prostaglandins Leukot Essent Fatty Acids. 1997. 57:473–477.

7. Rozen R. Genetic predisposition to hyperhomocysteinemia: deficiency of methylenetetrahydrofolate reductase (MTHFR). Thromb Haemost. 1997. 78:523–526.

8. Bertina RM, Koeleman BP, Koster T, Rosendaal FR, Dirven RJ, de Ronde H, et al. Mutation in blood coagulation factor V associated with resistance to activated protein C. Nature. 1994. 369:64–67.

9. Poort SR, Rosendaal FR, Reitsma PH, Bertina RM. A common genetic variation in the 3'-untranslated region of the prothrombin gene is associated with elevated plasma prothrombin levels and an increase in venous thrombosis. Blood. 1996. 88:3698–3703.

10. Cumming AM, Keeney S, Salden A, Bhavnani M, Shwe KH, Hay CR. The prothrombin gene variant: prevalence in a U.K. anticoagulant clinic population. Br J Haematol. 1997. 98:353–355.

11. Kapur RK, Mills LA, Spitzer SG, Hultin MB. A prothrombin gene mutation is significantly associated with venous thrombosis. Arterioscler Thromb Vasc Biol. 1997. 17:2875–2879.

12. Makris M, Preston FE, Beauchamp NJ, Cooper PC, Daly ME, Hampton KK, et al. Co-inheritance of the 20210A allele of the prothrombin gene increases the risk of thrombosis in subjects with familial thrombophilia. Thromb Haemost. 1997. 78:1426–1429.

13. Arruda VR, Annichino-Bizzacchi JM, Gonçalves MS, Costa FF. Prevalence of the prothrombin gene variant (nt20210A) in venous thrombosis and arterial disease. Thromb Haemost. 1997. 78:1430–1433.

14. Watzke HH, Schüttrumpf J, Graf S, Huber K, Panzer S. Increased prevalence of a polymorphism in the gene coding for human prothrombin in patients with coronary heart disease. Thromb Res. 1997. 87:521–526.

15. De Stefano V, Chiusolo P, Paciaroni K, Casorelli I, Rossi E, Molinari M, et al. Prothrombin G20210A mutant genotype is a risk factor for cerebrovascular ischemic disease in young patients. Blood. 1998. 91:3562–3565.

16. Rosendaal FR, Siscovick DS, Schwartz SM, Psaty BM, Raghunathan TE, Vos HL. A common prothrombin variant (20210 G to A) increases the risk of myocardial infarction in young women. Blood. 1997. 90:1747–1750.

17. Ferraresi P, Marchetti G, Legnani C, Cavallari E, Castoldi E, Mascoli F, et al. The heterozygous 20210 G/A prothrombin genotype is associated with early venous thrombosis in inherited thrombophilias and is not increased in frequency in artery disease. Arterioscler Thromb Vasc Biol. 1997. 17:2418–2422.

18. Corral J, Gonzalez-Conjero R, Lozano ML, Rivera J, Heras I, Vicente V. The venous thrombosis risk factor 20210A allele of the prothrombin gene is not a major risk factor for arterial thrombotic disease. Br J Haematol. 1997. 99:304–307.

19. Longstreth WT Jr, Rosendaal FR, Siscovic DS, Vos HL, Schwartz SM, Psaty BM, et al. Risk of stroke in young women and two prothrombotic mutations: factor VLeiden and Prothrombin gene variant (G20210A). Stroke. 1998. 29:577–580.

20. März W, Seydewitz H, Winkelmann B, Chen M, Nauck M, Witt I. Mutation in a coagulation factor V associated with resistance to activated protein C in patients with coronary artery disease. Lancet. 1995. 345:526.
21. Holm J, Zöller B, Berntorp E, Erhardt L, Dahlbäck B. Prevalence of factor V gene mutation amongst myocardial infarction patients and healthy controls is higher in Sweden than in other countries. J Intern Med. 1996. 239:221–226.

22. Rosendaal FK, Siscovick DS, Schwartz SM, Beverly RK, Psaty BM, Longstreth WT Jr, et al. Factor VLeiden (resistance to activated protein C) increases the risk of myocardial infarction in young women. Blood. 1997. 89:2817–2821.

23. van der Bom JG, Bots ML, Haverkate F, Slagboom PE, Meijer P, de Jong PT, et al. Reduced response to activated protein C is associated with increased risk for cerebrovascular disease. Ann Intern Med. 1996. 125:265–269.

24. Ridker PM, Hennekens CH, Lindpaintner K, Stampfer MJ, Eisenberg PR, Miletich JP. Mutation in the gene coding for coagulation factor V and the risk of myocardial infarction, stroke and venous thrombosis in apparently healthy men. N Engl J Med. 1995. 332:912–917.

25. Demarmels Biasiutti F, Merlo C, Furlan M, Sulzer I, Binder BR, Lämmle B. No association of APC resistance with myocardial infarction. Blood Coagul Fibrinolysis. 1995. 6:456–459.

26. Lerman A, Suwaidi JA, Velianou JL. L-Arginine: a novel therapy for coronary artery disease? Expert Opin Investig Drugs. 1999. 8:1785–1793.

27. Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993. 362:801–809.

28. Fuster V, Lewis A. Conner Memorial Lecture. Mechanisms leading to myocardial infarction: insights from studies of vascular biology. Circulation. 1994. 90:2126.

29. Farmer JA, Gotto AM Jr. Braunwa E, editor. Dyslipidemia and other risk factors for coronary artery disease. Heart disease. A textbook of cardiovascular medicine. 1997. 5th ed. Philadelphia, PA: W.B. Saunders.
30. Inbal A, Freimark D, Modan B, Chetrit A, Matetzky S, Rosenberg N, et al. Synergistic effects of prothrombic polymorphisms and atherogenic factors on the risk of myocardial infarction in young males. Blood. 1999. 93:2186–2190.

31. Russo C, Girelli D, Olivieri O, Guarini P, Manzato F, Pizzolo F, et al. G20210A prothrombin gene polymorphism and prothrombin activity in subjects with or without angiographically documented coronary artery disease. Circulation. 2001. 103:2436–2440.

32. Doggen CJ, Cats VM, Bertina RM, Rosendaal FR. Interaction of coagulation defects and cardiovascular risk factors: increased risk of myocardial infarction associated with factor VLeiden or prothrombin 20210A. Circulation. 1998. 97:1037–1041.

33. Burzotta F, Paciaroni K, De Stefano V, Chiusolo P, Manzoli A, Casorelli I, et al. Increased prevalence of the G20210A prothrombin gene variant in acute coronary syndromes without metabolic or acquired risk factors or with limited extent of disease. Eur Heart J. 2002. 23:26–30.

34. Makris TK, Krespi PG, Hatzizacharias AN, Gialeraki AE, Anastasiadis G, Triposkiadis FK, et al. Resistance to activated protein C and FV Leiden mutation in patients with a history of acute myocardial infarction or primary hypertension. Am J Hypertens. 2000. 13:61–65.

35. Ridker PM, Hennekens CH, Miletich JP. G20210A mutation in prothrombin gene and risk of myocardial infarction, stroke, and venous thrombosis in a large cohort of US men. Circulation. 1999. 99:999–1004.

36. Çelik S, Ovali E, Baykan M, Uçar F, Erdöl C, Durmuş I, et al. Factor VLeiden and its relation to left ventricular thrombus in acute myocardial infarction. Acta Cardiol. 2001. 56:1–6.

37. Juul K, Tybjaerg-Hansen A, Steffensen R, Kofoed S, Jensen G, Nordestgaard BG. Factor V Leiden: The Copenhagen City Heart study and 2 meta-analyses. Blood. 2002. 100:3–10.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download