Abstract
A 69-year-old male was diagnosed in February 2004 with stage IV extranodal marginal zone B cell lymphoma involving the mediastinal nodes, lung parenchyma and bone marrow with high LDH. Shortness of breath developed following the 5th course of Rituximab-CHOP chemotherapy (cyclophosphamide, Vincristine, Doxorubicin, Prednisolone). Bronchoscopy guided transbronchial lung biopsy revealed interstitial thickening and type II pneumocyte activation, compatible with interstitial pneumonitis. After treatment with prednisolone a complete resolution of the dyspnea was observed. The patient was well on routine follow-up at the outpatient clinic, with no progression of lymphoma or interstitial pneumonitis.
Rituximab, a chimeric anti-CD20 IgG1 monoclonal antibody, is an effective treatment for B-cell low grade lymphomas. This includes extranodal marginal zone B cell lymphoma,1-3 diffuse large B-cell lymphoma,4 refractory idiopathic thrombocytopenic purpura,5 and other autoimmune disorders.6
The proposed mechanism of action for this depletion includes antibody-dependent cell-mediated cytotoxicity, complement-dependent cytotoxicity, and direct induction of apoptosis.7
Interstitial pneumonitis and bronchiolitis obliterans with organizing pneumonia8-10 have been recently reported when using rituximab alone or in combination with other medications.
We report a case of interstitial pneumonitis in a patient with disseminated extranodal marginal zone B cell lymphoma after the 5th cycle of rituximab and CHOP.
A 69-year-old male was diagnosed in February 2004 with stage IV extranodal marginal zone B cell lymphoma involving the mediastinal nodes, lung parenchyma and bone marrow.
Past medical history included one vessel disease, for which the patient underwent percutaneous coronary angioplasty; diabetes mellitus, and hypercholesterolemia. Medications included glimepiride, aspirin, isosorbide dinitrate, nicoradil, and atorvastatin. None of these drugs had been taken since the diagnosis of non-Hodgkin's lymphoma.
Following the 5th course of rituximab-CHOP chemotherapy the patient was admitted with neutropenic fever accompanied by generalized weakness and mucositis.
Empiric broad spectrum antibiotics were started along with granulocyte-colony stimulating factor. Despite recovery of the neutrophil count, the shortness of breath was aggravated and arterial blood gas analysis revealed hypoxemia (PaO2=55).
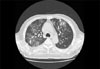
Gram stain and special stains of the sputum for acid-fast bacilli, pneumocystis carinii, and fungi were negative. Routine cultures for bacteria, fungi and mycobacteria yielded no growth. A chest X-ray showed progressive infiltrates in both lung fields. A computed tomography (CT) scan of the lung revealed bilateral patchy ground-glass opacities, suggestive of new onset interstitial pneumonitis. CT also showed a reduced primary mass in the right middle lobe, consistent with the initial primary lesion (Fig. 1).
Extrathoracic echocardiogram showed normal left ventricular contractility with unaltered ejection fraction. Cardiac enzymes were also normal. Ventilation perfusion scan showed no evidence of pulmonary artery embolism. Fiberoptic bronchoscopy revealed no evidence of endobronchial lesion. No malignant cells were found in the bronchoalveolar lavage fluid.
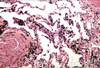
Gram stains, special analysis and cell cultures obtained from BAL and sputum following fiberoptic bronchoscopy were negative for bacteria, acid-fast bacilli, fungi and pneumocystis carinii. Bronchoscopy guided transbronchial lung biopsy was performed, showing interstitial thickening and type II pneumocyte activation compatible with interstitial pneumonitis (Fig. 2).
The patient was treated with prednisolone, 1 mg/kg a day, for 2 weeks. The dyspnea and tachypnea gradually improved, in addition to blood oxygenation and chest X-ray. A subsequent CT scan following prednisolone treatment showed marked improvement of the multifocal patchy interstitial infiltration and ground glass opacities; as well as a reduction in the primary lymphoma lesion.
After 42 days of hospitalization, the patient was discharged on a tapering prednisolone regimen. He experienced complete resolution of dyspnea and dyspnea on exertion. He was well on routine follow-up at the outpatient clinic, with no lymphoma progression or interstitial pneumonitis.
The optimal therapeutic management for patients with non-gastric extranodal marginal zone B cell lymphoma has not yet been defined. For localized disease, local treatment (either radiotherapy or surgery) will typically achieve excellent disease control.11-13 When the disease is advanced or disseminated, several chemotherapeutic agents alone in combination are usually administered. These regimens include alkylating agents, anthracyclins, steroids, and purine analogs.2,3,13 Anthracylines-based chemotherapy is typically recommended in patients with histological transformation or with high tumor mass (i.e. high LDH or a mass greater than 7cm).13
The use of rituximab alone or combination with cytotoxic chemotherapeutic agents is a new treatment breakthrough in disseminated or refractory disease. This is because extranodal marginal zone B cells typically express the CD20 antigen.13,14
Adverse pulmonary reactions to rituximab occur as reversible events during the first infusion in 38% of patients. In addition, severe human antibody infusion reactions, generally occurring during the first 2 hours of the first infusion, can present with hypotension or angioedema. Pulmonary involvement manifests in the form of bronchospasm, hypoxia, infiltrates and even acute respiratory distress syndrome.15 Late occurring pulmonary toxicities after the administration of rituximab have rarely been reported.8-10,15-18
Mechanisms leading to rituximab-induced interstitial lung disease are not yet completely elucidated. However, dysregulated cellular cytotoxicity may be related to a mechanism of delayed pulmonary toxicity.17,19 Cytotoxic T lymphocytes (CTL) are believed to play an important role in long term anti-tumor effects after rituximab treatment.17,19 Their activation ('cross-priming') appears to be induced by dendritic cells, which mature under the influence of cell-derived peptides resulting from rituximab-induced tumor destruction.17,19 CTL activation may produce vascular and alveolar damage. Disturbed cellular cytotoxicity can also result from the interaction of rituximab with CD 20+ T-cells,17,20-22 or by cross-reactivity between lung and tumoral antigens with possible generation of a self reactive clone.17
Pulmonary leukostasis is associated with activation of the complement system and changes in endothelial adhesivity induced by elevated cytokine levels. This leukostasis may also contribute to the dyspnea and bronchospasm often encountered in patients with high tumor burden after treatment with rituximab.17,23,24
The emergence of pulmonary infiltrates in patients treated with rituximab and CHOP chemotherapy remains a difficult diagnostic problem. Many differential diagnoses need to be considered, including lymphoma progression, infection, cardiogenic edema, radiation pneumonitis, pulmonary hemorrhage, and allergies.
One of the major clinical challenges in these patients is ruling out opportunistic infection or lymphoma progression. The infectious etiologies of diffuse interstitial infiltrates in the lungs are multiple.25 They include any Gram-negative or Gram-positive agents, fungi (Aspergillus, Candida), parasites (Pneumocystis carinii, Toxoplasma gondii), or viruses (Herpes simplex, Varicella zoster, Cytomegalovirus).25
Non-invasive tests and studies such as chest X-ray, CT scan, echocardiogram, pulmonary function tests, and arterial blood gas analysis can easily determine the pattern of pulmonary disease present. However, fiberoptic bronchoscopy with bronchoalveolar lavage and lung biopsy is usually necessary to eliminate infection or lymphoma progression. The exclusion of all known etiologic factors associated with pulmonary interstitial infiltrates is essential for diagnosing rituximab-induced interstitial pneumonitis.
We conclude that physicians should be aware of this rare, but possible complication of interstitial pneumonitis after combination rituximab-CHOP chemotherapy.
Figures and Tables
References
1. Hiddemann W, Kneba M, Dreyling M, Schmitz N, Lengfelder E, Schmits R, et al. Frontline therapy with rituximab added to the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) significantly improves the outcome for patients with advanced-stage follicular lymphoma compared with therapy with CHOP alone: results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood. 2005. 106:3725–3732.

2. Ahmed S, Kussick SJ, Siddiqui AK, Bhuiya TA, Khan A, Sarewitz S, et al. Bronchial-associated lymphoid tissue lymphoma: a clinical study of a rare disease. Eur J Cancer. 2004. 40:1320–1326.

3. Conconi A, Martinelli G, Thieblemont C, Ferreri AJ, Devizzi L, Peccatori F, et al. Clinical activity of rituximab in extranodal marginal zone B-cell lymphoma of MALT type. Blood. 2003. 102:2741–2745.

4. Coiffier B. State-of-the-art therapeutics: diffuse large B-cell lymphoma. J Clin Oncol. 2005. 23:6387–6393.

5. Braendstrup P, Bjerrum OW, Nielsen OJ, Jensen BA, Clausen NT, Hansen PB, et al. Rituximab chimeric anti-CD20 monoclonal antibody treatment for adult refractory idiopathic thrombocytopenic purpura. Am J Hematol. 2005. 78:275–280.

6. Higashida J, Wun T, Schmidt S, Naguwa SM, Tuscano JM. Safety and efficacy of rituximab in patients with rheumatoid arthritis refractory to disease modifying antirheumatic drugs and anti-tumor necrosis factor-alpha treatment. J Rheumatol. 2005. 32:2109–2115.
7. Maloney DG, Smith B, Rose A. Rituximab: mechanism of action and resistance. Semin Oncol. 2002. 29(1 Suppl 2):2–9.

8. Kanelli S, Ansell SM, Habermann TM, Inwards DJ, Tuinstra N, Witzig TE. Rituximab toxicity in patients with peripheral blood malignant B-cell Lymphocytosis. Leuk Lymphoma. 2001. 42:1329–1337.

9. Burton C, Kaczmarski R, Jan-Mohamed R. Interstitial pneumonitis related to rituximab therapy. N Engl J Med. 2003. 348:2690–2691.

10. Byrd JC, Peterson BL, Morrison VA, Park K, Jacobson R, Hoke E, et al. Randomized phase 2 study of fludarabine with concurrent versus sequential treatment with rituximab in symptomatic, untreated patients with B-cell chronic lymphocytic leukemia: results from Cancer and Leukemia Group B 9712 (CALGB 9712). Blood. 2003. 101:6–14.

11. Tsang R, Gospodarowicz MK, Pintilie M, Bezjak A, Wells W, Hodgson DC, et al. Stage I and II MALT lymphoma: results of treatment with radiotherapy. Int J Radiat Oncol Biol Phys. 2001. 50:1258–1264.

12. Schechter NR, Portlock CS, Yahalom J. Treatment of mucosa-asociated lymphoid tissue lymphoma of the stomach with radiation alone. J Clin Oncol. 1998. 16:1916–1921.

13. Thieblemont C. Clinical presentation and management of marginal zone lymphomas. Hematology Am Soc Hematol Educ Program. 2005. 307–313.

14. Soda R, Costanzo A, Cantonetti M, Orlandi A, Bianchi L, Chimenti S. Systemic therapy of primary cutaneous B-cell lymphoma, marginal zone type, with rituximab, a chimeric anti-CD20 monoclonal antibody. Acta Derm Venereol. 2001. 81:207–208.

15. Lee Y, Kyung SY, Choi SJ, Bang SM, Jeong SH, Shin DB, et al. Two cases of interstitial pneumonitis caused by rituximab therapy. Korean J Intern Med. 2006. 21:183–186.

16. Hainsworth JD, Litchy S, Lamb MR, Rodriguez GI, Scroggin C Jr, Greco FA. First-line treatment with brief-duration chemotherapy plus rituximab in elderly patients with intermediate-grade non-Hodgkin's lymphoma: phase II trial. Clin Lymphoma. 2003. 4:36–42.

17. Alexandrescu DT, Dutcher JP, O'Boyle K, Albulak M, Oiseth S, Wiernik PH. Fatal intra-alveolar hemorrhage after rituximab in a patient with non-Hodgkin lymphoma. Leuk Lymphoma. 2004. 45:2321–2325.

18. Herishanu Y, Polliack A, Leider-Trejo L, Grieff Y, Metser U, Naparstek E. Fatal interstitial pneumonitis related to rituximab-containing regimen. Clin Lymphoma Myeloma. 2006. 6:407–409.

19. Selenko N, Maidic O, Draxier S, Berer A, Jäger U, Knapp W, et al. CD20 antibody (C2B8)-induced apoptosis of lymphoma cells promotes phagocytosis by dendritic cells and cross-priming of CD8+ cytotoxic T cells. Leukemia. 2001. 5:1619–1626.

20. Hultin LE, Hausner MA, Hultin PM, Giorgi JV. CD20 (pan-B cell) antigen is expressed at a low level on a subpopulation of human T lymphocytes. Cytometry. 1993. 14:196–204.

21. Algino KM, Thomason RW, King DE, Montiel MM, Craig FE. CD20 (pan-B cell antigen) expression on bone marrow-derived T cells. Am J Clin Pathol. 1996. 106:78–81.

22. Warzynski MJ, Graham DM, Axtell RA, Zakem MH, Rotman RK. Low level CD20 expression on T cell malignancies. Cytometry. 1994. 18:88–92.

23. Jensen M, Winkler U, Manzke O, Diehl V, Engert A. Rapid tumor lysis in a patient with B-cell chronic lymphocytic leukemia and lymphocytosis treated with an anti-CD20 monoclonal antibody (IDEC-C2B8, rituximab). Ann Hematol. 1998. 77:89–91.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download