Abstract
We present an adult female patient who developed irreversible paraplegia and areflexia four days post intrathecal chemotherapy with methotrexate, cytosine arabinoside and hydrocortisone. On magnetic resonance imaging (MRI) of the lumbar spine, diffuse gadolinium enhancement of the anterior spinal nerve roots (ventral roots) was detected. Methylprednisolone was intravenously administered at a daily dose of 30mg/kg for three days. Despite this treatment, flaccid weakness in the lower extremities and urinary retention persisted. Following consolidation chemotherapy, no improvement in neurologic status was noted. Six months later, a follow-up MRI revealed severe atrophy of the thoracic spinal cord.
A standard approach for central nervous system (CNS) prophylaxis in patients with acute lymphoblastic leukemia (ALL) is the use of intrathecal chemotherapy with methotrexate (MTX) or cytosine arabinoside (Ara-C).1
More patients are surviving ALL; and complications have accordingly increased in number due to the adverse effects of intrathecal chemotherapy. Chemical arachidonitis, myelopathy and leukoencephalopathy are the most common adverse effects of intrathecal chemotherapy. However, to our knowledge studies have reported fewer incidents of myelopathy following intrathecal chemotherapy in adult patients with ALL than in their pediatric counterparts.2,3
We recently encountered a case of ALL in remission in which irreversible paraplegia was noted after a single dose of prophylactic intrathecal chemotherapy with MTX, Ara-C and hydrocortisone. We evaluated the clinical, MRI and electrodiagnostic findings in this case.
A 25-year-old female patient was admitted in February of 2004, complaining of generalized easy bruising and weakness. Initial CBC showed bicytopenia: Hb 4.8g/dL, platelets 21,000/µL and WBC 14,280/µL (75% blasts). An immunophenotype was characterized by the expression of CD19 (89%) and CD22 (83.3%), in which HLA-DR (94%) and CD34 (99%) were strong. A bone marrow examination showed approximately 90% small-to-medium sized blasts with a small amount of blue cytoplasm.
In a chromosomal analysis of the bone marrow a 46, XX karyotype was found in 100% (20/20). RT-PCR analysis was negative for Bcr/Abl rearrangement. Based on these findings, we made the diagnosis of precursor B-cell ALL in our patient. Induction chemotherapy of the VPDL regimen consisted of 2mg intravenous vincristine on days 1, 8, 15 and 22; 60mg/m2 prednisolone on days 1 through 14; 45mg/m2 intravenous daunorubicin on days 1 to 3; and 4,000units/m2 intramuscular L-asparaginase on days 17 to 28. On day 42, bone marrow examination showed complete hematologic remission was achieved.
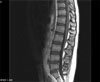
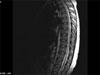
On day 43, our patient received prophylactic intrathecal chemotherapy via lumbar puncture. This consisted of preservative-free 12mg MTX, 40 mg cytarabine and 50mg hydrocortisone. Four days later, she was noted to have leg weakness, and symptoms progressed to the point that she could not walk. In addition, the patient could not move her legs without gravity. She had no deep tendon reflexes over her patellae or ankles, and exhibited a mild sensory deficit. We examined the first CSF cytology concurrently with the prophylactic intrathecal chemotherapy, and identified atypical lymphocytes which were suspected to be blasts. On the second, third and fourth CSF cytologies however; no leukemic involvement was evidenced. No further intrathecal chemotherapy was given to the patient. Electromyography (EMG) and nerve conduction velocity (NCV) studies were consistent with the presence of myelopathy and neuropathy in the motor and left median axons. On magnetic resonance imaging (MRI) of the lumbar spine, diffuse gadolinium enhancement of the anterior roots (ventral roots) was detected (Fig. 1). Methylprednisolone was administered at a daily dose of 30mg/kg for three days, however lower extremity weakness and urinary retention persisted. Eight months after the first MRI scan, following nine cycles of consolidation chemotherapy, a follow-up T-2 weighted MRI image was taken. This revealed heterogeneous high intensity lesions in the thoracic spinal cord, in addition to severe atrophy (Fig. 2). The patient was in complete hematologic remission and was given oral MTX, 6-mercaptopurine and prednisolone for maintenance chemotherapy. However she demonstrated no improvement in her paraplegia and areflexia 18 months following the first signs of irreversible paraplegia.
Intrathecal chemotherapy with MTX, Ara-C, or both with or without hydrocortisone is considered the standard of care for prophylaxis and treatment of CNS leukemia and lymphoma.1
According to several studies, intrathecal chemotherapy with MTX or Ara-C can lead to paraplegia, with accompanying encephalopathy in certain cases.2-18
Little is known about the mechanism by which intrathecal chemotherapy causes neurologic complications. Speculation suggests they are related to the neurotoxicity of MTX per se, or preservatives added as a diluent. A local depletion of folate secondary to MTX therapy or a high CSF level of MTX might also be attributable factors.19-21 Additionally, Ara-C has a longer half-life in the CSF as compared to plasma. This property is related to the lowered activity of Ara-C deaminase in the CSF and the spinal cord, and may further explain the neurotoxicity of Ara-C.
Intrathecal chemotherapy with MTX and Ara-C could in theory cause myelopathy, although this was rarely reported in adult patients with ALL.22
In existing literature, patients had a history of multiple intrathecal MTX injections (5-53 times) prior to developing paraplegia. The range for a single intrathecal dose of MTX was 5-25mg. The limiting cumulative dose varied from 90 and 305 mg.8 However, irreversible paraplegia occurred after a single dose of intrathecal chemotherapy in our patient.
Literature suggests that the concomitant use of intravenous or intrathecal Ara-C with intrathecal MTX may enhance the neurotoxicity of MTX. The dosage range of Ara-C in concomitant use with intrathecal MTX is usually 30-170mg, while the toxic cumulative dose of Ara-C ranges between 40 and 780mg.8
To provoke the potential lymphotoxic effect as well as to reduce arachnoiditis and other inflammations of the CNS, hydrocortisone is often administered concomitantly with intrathecal MTX or Ara-C. However, to date no studies have proven that concomitant use of hydrocortisone prevents myelopathy.23
In this case concomitant usage of Ara-C with MTX was the lone precipitating factor. Neuropathologic findings of myelopathy following intrathecal chemotherapy typically include demyelination, microvacuolization and scattered axonal swelling in the spinal cord.6,18,23,24 In cases of myelopathy, MRI is the most sensitive diagnostic modality for detection of signal abnormalities of the spinal cord.
In cases of myelopathy following intrathecal chemotherapy, treatment choices are made in a limited scope. However, it is always imperative to cease further intrathecal chemotherapy.
Myelopathy following intrathecal chemotherapy is a rare but disastrous complication, and the mechanism involved is not clearly understood. In the management of adult patients with ALL physicians must make a diagnosis of exclusion, ruling out other potential etiologies such as infection or leptomeningeal seeding.
Figures and Tables
References
1. Bleyer WA, Poplack DG. Prophylaxis and treatment of leukemia in the central nervous system and other sanctuaries. Semin Oncol. 1985. 12:131–148.
2. Bay A, Oner AF, Etlik O, Yilmaz C, Caksen H. Myelopathy due to intrathecal chemotherapy: report of six cases. J Pediatr Hematol Oncol. 2005. 27:270–272.
3. Skullerud K, Halvorsen K. Encephalomyelopathy following intrathecal methotrexate treatment in a child with acute leukemia. Cancer. 1978. 42:1211–1215.

4. Boogerd W, Moffie D, Smets LA. Early blindness and coma during intrathecal chemotherapy for meningeal carcinomatosis. Cancer. 1990. 65:452–457.

5. Breuer AC, Pitman SW, Dawson DM, Schoene WC. Paraparesis following intrathecal cytosine arabinoside: a case report with neuropathologic findings. Cancer. 1977. 40:2817–2822.

6. Clark AW, Cohen SR, Nissenblatt MJ, Wilson SK. Paraplegia following intrathecal chemotherapy: neuropathologic findings and elevation of myelin basic protein. Cancer. 1982. 50:42–47.

7. Dunton SF, Nitschke R, Spruce WE, Bodensteiner J, Krous HF. Progressive ascending paralysis following administration of intrathecal and intravenous cytosine arabinoside. A Pediatric Oncology Group Study. Cancer. 1986. 57:1083–1088.

8. Gagliano RG, Costanzi JJ. Paraplegia following intrathecal methotrexate: report of a case and review of the literature. Cancer. 1976. 37:1663–1668.

9. Grisold W, Lutz D, Wolf D. Necrotizing myelopathy associated with acute lymphoblastic leukemia. Case report and review of literature. Acta Neuropathol. 1980. 49:231–235.

10. Luddy RE, Gilman PA. Paraplegia following intrathecal methotrexate. J Pediatr. 1973. 83:988–992.

11. Mena H, Garcia JH, Velandia F. Central and peripheral myelinopathy associated with systemic neoplasia and chemotherapy. Cancer. 1981. 48:1724–1737.

12. Norman M, Elinder G, Finkel Y. Vincristine neuropathy and a Guillain-Barré syndrome: a case with acute lymphatic leukemia and quadriparesis. Eur J Haematol. 1987. 39:75–76.

13. Ojeda VJ. Necrotizing myelopathy associated with malignancy. A clinicopathologic study of two cases and literature review. Cancer. 1984. 53:1115–1123.

14. Resar LM, Phillips PC, Kastan MB, Leventhal BG, Bowman PW, Civin CI. Acute neurotoxicity after intrathecal cytosine arabinoside in two adolescents with acute lymphoblastic leukemia of B-cell type. Cancer. 1993. 71:117–123.

15. Saiki JH, Thompson S, Smith F, Atkinson R. Paraplegia following intrathecal chemotherapy. Cancer. 1972. 29:370–374.

16. Werner RA. Paraplegia and quadriplegia after intrathecal chemotherapy. Arch Phys Med Rehabil. 1988. 69:1054–1056.
17. Wolff L, Zighelboim J, Gale RP. Paraplegia following intrathecal cytosine arabinoside. Cancer. 1979. 43:83–85.

18. Koh S, Nelson MD Jr, Kovanlikaya A, Chen LS. Anterior lumbosacral radiculopathy after intrathecal methotrexate treatment. Pediatr Neurol. 1999. 21:576–578.

19. Gilbert MR, Harding BL, Grossman SA. Methotrexate neurotoxicity: In vitro studies using cerebellar explants from rats. Cancer Res. 1989. 49:2502–2505.
20. Watterson J, Toogood I, Nieder M, Morse M, Frierdich S, Lee Y, et al. Excessive spinal cord toxicity from intensive central nervous system-directed therapies. Cancer. 1994. 74:3034–3041.

21. Bleyer WA, Drake JC, Chabner BA. Neurotoxicity and elevated cerebrospinal-fluid methotrexate concentration in meningeal leukemia. N Engl J Med. 1973. 289:770–773.

22. Sherman PM, Belden CJ, Nelson DA. Magnetic resonance imaging findings in a case of cytarabine-induced myelopathy. Mil Med. 2002. 167:157–160.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download