Abstract
Purpose
This study was undertaken to determine the effects of intratracheal administration of endotoxin on hyperoxia-induced lung injury in neonatal rats.
Materials and Methods
Newborn Sprague Dawley rat pups were divided into four experimental groups: normoxia control (NC), normoxia with endotoxin treatment (NE), hyperoxia control (HC), and hyperoxia with endotoxin treatment (HE) groups. In HC and HE, rat pups were subjected to 14 days of hyperoxia (> 95% oxygen) within 12 hours after birth. In endotoxin treated group (NE and HE), Escherichia coli endotoxin (0.5µg in 0.03mL of saline) was given intratracheally at the 1st, 3rd and 5th postnatal day. Radial alveolar count (RAC), mean linear intercept (MLI), RAC/MLI ratios, and degree of fibrosis were measured to assess the changes in lung morphology.
Results
During the research period, survival rates in both HC and HE were notably reduced 7 days after endotoxin was administered, but body weight gain was considerably reduced only in HC. On day 14, significant arrest in alveolarization, as evidenced by the decrease of RAC and RAC/MLI ratio and increase of MLI as well as increased fibrosis, were noted in HC. Although slight but significant arrest in alveolarization and increased fibrosis score were observed in NE compared to NC, the hyperoxia-induced lung damage observed in HC was significantly improved in HE.
Bronchopulmonary dysplasia (BPD), a form of chronic lung disease usually occurring in premature infants, is an important cause of mortality and morbidity during the neonatal period with few effective treatment.1 Histopathologic characteristics of BPD include airway injury, inflammation, and parenchymal fibrosis.2-4 The precise mechanism of BPD has not yet been elucidated, therefore, it was felt of necessity to better understand its pathophysiologic mechanism to develop therapeutic modality that can prevent lung injury and consequently improve the prognosis of BPD.
Clinically, antenatal inflammation, diagnosed by chorioamnionitis or by elevated cytokines in amniotic fluid, is associated with increased risks of preterm delivery and BPD.5 Neonatal sepsis and/or pneumonia also are known to be risk factors of BPD.6 However, chorioamnionitis has been associated with decreased incidence of respiratory distress syndrome7 and improved survival.8 In experimental animals, lung inflammation can be produced by administering endotoxin, a lipopolysaccharide component of Gram-negative bacteria.9,10 Intra-alveolar inflammatory cell recruitment and/or reduced alveolar growth were observed after intratracheal11 or intraamniotic endotoxin administration.12 However, systemic administration of endotoxin significantly protected lungs against oxygen toxicity in neonatal rats.13 Therefore, available data on the role of inflammation due to infection in the development of BPD remain dubious, and further studies are needed to clarify this. This study was done to determine the effects of intratracheal administration of endotoxin on hyperoxia-induced lung injury in neonatal rats.
The experimental protocols described herein were reviewed and approved by the Animal Care and Use Committee of the Samsung Biomedical Research Institute in Seoul, Korea. This study also followed the institutional and National Institutes of Health guidelines for laboratory animal care. Timed pregnant Sprague Dawley rats were housed in individual cages with free access to water and laboratory chow, and pups were delivered spontaneously. The experiment began within 12 hours after birth and was continued through postnatal day 14.
Rat pups were divided into four experimental groups: normoxia control group (NC, n=21), normoxia with endotoxin treatment group (NE, n=24), hyperoxia control group (HC, n=46), and hyperoxia with endotoxin treatment group (HE, n=48). Throughout the experiment, rat pups of NC and NE were kept in standard cages with nursing mother rat at room air, and pups of HC and HE were kept in standard cages with mother rat within 50 liter Plexiglas chamber in which hyperoxia (> 95% oxygen concentration) was maintained. Humidity was maintained at 50%, and environmental temperature was maintained at 22-24℃. To avoid oxygen toxicity of nursing mother rat, mother rats were rotated daily between litters in normoxia groups and hyperoxia groups.
Pups in NE and HE were injected with E. coli endotoxin (0.5µg in 0.03mL, Lipopolysaccharide, LPS from Escherichia coli O55:B5; Sigma, St Louis, Mo; 10mg) intratracheally at the 1st, 3rd and 5th postnatal day. Rats were restrained on a board on a fixed angle, neck was palpated to locate the trachea, and 1cc syringe with a 30-gauge needle was introduced into the trachea for instillation of endotoxin. Intratracheal location of the needle was confirmed by withdrawal of air into the syringe with minimal reflux of suspension back through the nose. Air was pulsed after each liquid volume. Pups in NC and HC received an equal volume of normal saline administered in the same way. Animals were returned to their dams after the procedure, and there was no mortality associated with the procedure.
Survival and body weight of rat pups in each group were checked daily throughout the experiment, and were sacrificed at postnatal day 14 after intraperitoneal injection of ketamine (Yuhan Corp., Seoul, Korea, 50mg/kg).
After perfusion of the heart with normal saline, lungs were fixed in situ at a constant inflation pressure of 20cm H2O and fixed overnight in 10% buffered formalin. The fixed lung tissue was embedded in paraffin wax after tissue processing. Five µm-thick sections were cut from the paraffin block and stained with hematoxylin and eosin. Images of each section were captured with Baumer optronic digital camera through an Olympus BX40 microscope (Olympus optical co. Ltd., Tokyo, Japan) and were saved as JPEG files.
Alveolarization was assessed by performing radial alveolar counts (RAC), according to the method of Emery and Mithal.14,15 From the center of the respiratory bronchiole, a perpendicular line was drawn to the edge of the acinus (as defined by a connective tissue septum or the pleura), and the number of the septa intersected by this line was counted.
The intra-alveolar distance was measured as the mean linear intercept (MLI), obtained by dividing the total length of lines drawn across the lung section by the number of intercepts encountered, as described by Cooney and Thurlbeck.16 After obtaining the RAC and MLI values, the RAC/MLI ratio was calculated in each case.
Each successive lung field was individually assessed for severity of interstitial fibrosis and allotted a score 0 and 8, as described by Ashcroft et al.17 A minimum of 10 lung fields per section was examined for each analysis.
All data are presented as mean±standard deviation. Statistical comparison between each group was performed by one-way analysis of variance with Bonferroni's correction. For comparison of survival curves, Kaplan-Meiyer analysis was performed. A p-value of < 0.05 was considered significant.
At the end of the experiment (postnatal day 14), exposure to oxygen groups significantly decreased the survival rate (HC and HE, 48% and 58%, respectively) compared to normoxia groups (NC and NE, 89% and 75%, respectively) (Fig. 1).
Although birth weights were not different between the four experimental groups, pups nursed in hyperoxia alone (HC) showed significant decrease in body weight compared to normoxia groups (NC, NE) after postnatal day 7, and this hyperoxia-induced decrease of body weight was improved with endotoxin administration (Fig. 2).
Representative photomicrographs showing histopathologic differences in each experimental group are shown in Fig. 3. Morphometric analyses revealed slight but significant decrease in RAC and RAC/MLI ratio and increased fibrosis score in NE compared to NC (Fig. 4, 5). Compared to normoxia groups (NC, NE), RAC and RAC/MLI ratio in HC were significantly decreased, whereas MLI and fibrosis score were significantly increased. These hyperoxia-induced lung morphometric abnormalities, however, were significantly attenuated with endotoxin administration (HE).
The development of an appropriate animal model that can simulate clinical BPD of premature infants is essential for delineation of its pathophysiologic mechanism. The saccular stage of the newly born rat pup corresponds to the lung developmental stage of premature infants at 25-28 weeks gestation18 and alveolarization occurs during the first 2 weeks after birth.19 Hence, the newborn rat pup model is a model very suitable for studying pathophysiologic mechanisms of BPD. In the histopathologic study of the lung, Husain et al.20 reported that RAC, MLI, and their ratios are simple and the most useful way of studying acinar growth and development.
Oxidative stress to the immature lung is a well-known risk factor for the development of BPD.21-23 In the present study, prolonged exposure of newborn rat pups to hyperoxia developed lung injuries similar to those seen in human infants with BPD,2 exhibiting decreased alveolarization as evidenced by decreased RAC, increased MLI3 and markedly increased fibrosis.4
Mild arrest in alveolar growth that was evidenced by a slight decrease in the RAC and RAC/MLI ratio, and an increase in fibrosis was also observed in the endotoxin treated normoxia group (NE) compared to NC. These findings strengthen the assumption that inflammation due to infection can disrupt alveolar development in preterm lung, causing histological changes similar to BPD.12,13,24,25 However, endotoxin administration during hyperoxia (HE) significantly attenuated hyperoxia-induced abnormalities, such as an increase in MLI and fibrosis score, decrease in RAC, RAC/MLI ratio, and body weight. Taken together, our data indicate that the friend or foe simple dichotomy could not account for the complex role of inflammation induced by endotoxin in the pathogenesis of BPD.
Although hyperoxia-induced mortality in the present study was not significantly improved, growth retardation observed in HC was significantly attenuated with endotoxin treatment (HE). Growth retardation observed in HC might be attributable to higher caloric requirements of rat pups with more severe lung disease. More severe pathologic findings and clinical symptoms of BPD, such as dyspnea and tachypnea, in HC than in HE support our speculation.
The precise mechanisms by which endotoxin attenuates hyperoxia-induced lung injury are not completely understood. The protective effects of endotoxin might be attributable to a combination of enhanced lung maturation,26,27 increased antioxidants,13,28 surfactant,12,29,30,31 loss of L-selectin and reduced neutrophils in the lung,32 and increased anti-inflammatory cytokines such as interleukin 11 and 13.33
Significant attenuation of the hyperoxia-induced lung injury with endotoxin treatment observed in this study contradicts the results by Kohno et al. who reported that the most remarkable emphysematous changes were noted in endotoxin plus hyperoxia group.34 As 10-week old rats were used in the above study compared to 1-day-old newborn rat pups in this study, differences in the stage of lung maturation might be responsible for these discrepancies with endotoxin treatment.
In the present study, endotoxin was given intratracheally. Since the protective effect has also been observed against the hyperoxia-induced lung injury with systemic (intraperitoneal) administration of endotoxin,13 the protective effects of endotoxin appear to be not influenced by the routes of administration.
Despite such effects of endotoxin, low survival rate was noted in HC and HE groups on day 14 of the study, and this could be explained by relatively high death rate in the endotoxin-administered groups (NE and HE) during the earlier study period. It is highly likely that the concentration of endotoxin was higher than that used in earlier studies, and that there was some leakage into the systemic circulation.
The results described herein led us to conclude that the administration of endotoxin, a potent proinflammatory mediator, significantly attenuated hyperoxia-induced neonatal lung injury as evidenced by improvement in the arrest of alveolarization and in the increased fibrosis. These findings do not support the assumption that antenatal inflammation due to infection causes BPD, instead suggesting some beneficial effects of chorioamnionitis.
Figures and Tables
 | Fig. 1Time course of survival in each experimental group in 95% oxygen or room air for 14 days. Normoxia control group (NC), normoxia with endotoxin treatment group (NE), hyperoxia control group (HC), hyperoxia with endotoxin treatment group (HE). Values represent group means±SD. *p < 0.05 vs NC. |
 | Fig. 2Time course of weight change of endotoxin instillation and control group rat pups in > 95% oxygen or room air for 14 days. Normoxia control group (NC), normoxia with endotoxin treatment group (NE), hyperoxia control group (HC), hyperoxia with endotoxin treatment group (HE). Values represent group means±SD. *p < 0.05 vs NC. |
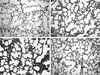 | Fig. 3Representative light micrographs of lung of rat pups at postnatal day 14. (A) Lung structure of neonatal rats in normoxia at day 14 (NC). Note prominent secondary alveolar septa formation. (B) Intratracheal instillation of endotoxin (NE) shows less prominent secondary alveolar septa formation and slightly alveolar dilatation. (C) Hyperoxia causes distal air space enlargement, and alveolar architecture is simplified (HC) and prominent fibrosis. (D) Intratracheal instillation of endotoxin during hyperoxia (HE), significantly attenuated hyperoxia-induced lung injury (Hematoxylin-eosin stain, original magnification ×100). |
 | Fig. 4Radial alveolar counts (A), mean linear intercepts (B), and radial alveolar counts/mean linear intercepts ratio (C) at postnatal day 14 in four treatment groups: normoxia control group (NC), normoxia with endotoxin treatment group (NE), hyperoxia control group (HC), hyperoxia with endotoxin treatment group (HE). Columns are means±SD. *p < 0.05 vs NC, †p < 0.05 vs NE, ‡p < 0.05 vs HC. |
 | Fig. 5Effect of hyperoxia and endotoxin on fibrosis at post-natal day 14 rat lung. Top: representative photomicrographs (×400 magnification) for each condition. Bottom: mean fibrosis scores of each group; normoxia control group (NC), normoxia with endotoxin treatment group (NE), hyperoxia control group (HC), hyperoxia with endotoxin treatment group (HE). Values represent group means±SD. *p < 0.05 vs NC; †p < 0.05 vs NE; ‡p < 0.05 vs HC. |
References
1. Abman SH, Groothius JR. Pathophysiology and treatment of bronchopulmonary dysplasia. Current issues. Pediatr Clin North Am. 1994. 41:277–315.

2. Saugstad OD. Bronchopulmonary dysplasia-oxidative stress and antioxidants. Semin Neonatol. 2003. 8:39–49.

3. Ghezzi F, Gomez R, Romero R, Yoon BH, Edwin SS, David C, et al. Elevated interleukin-8 concentrations in amniotic fluid of mothers whose neonates subsequently develop bronchopulmonary dysplasia. Eur J Obstet Gynecol Reprod Biol. 1998. 78:5–10.

4. Jobe AH, Ikegami M. Mechanisms initiating lung injury in the preterm. Early Hum Dev. 1998. 53:81–94.

5. Kallapur SG, Jobe AH. Contribution of inflammation to lung injury and development. Arch Dis Child Fetal Neonatal Ed. 2006. 91:F132–F135.

6. Rojas MA, Gonzalez A, Bancalari E, Claure N, Poole C, Silva-Neto G. Changing trends in the epidemiology and pathogenesis of neonatal chronic lung disease. J Pediatr. 1995. 126:605–610.

7. Watterberg KL, Demers LM, Scott SM, Murphy S. Chorioamnionitis and early lung inflammation in infants in whom bronchopulmonary dysplasia develops. Pediatrics. 1996. 97:210–215.

8. Costeloe K, Hennessy E, Gibson AT, Marlow N, Wilkinson AR. The EPICure study: outcomes to discharge from hospital for infants born at the threshold of viability. Pediatrics. 2000. 106:659–671.

9. Kramer BW, Kramer S, Ikegami M, Jobe AH. Injury, inflammation, and remodeling in fetal sheep lung after intra-amniotic endotoxin. Am J Physiol Lung Cell Mol Physiol. 2002. 283:L452–L459.
10. Ueda K, Cho K, Matsuda T, Okajima S, Uchida M, Kobayashi Y, et al. A rat model for arrest of alveolarization induced by antenatal endotoxin administration. Pediatr Res. 2006. 59:396–400.

11. Franco ML, Waszak P, Banalec G, Levame M, Lafuma C, Harf A, et al. LPS-induced lung injury in neonatal rats: changes in gelatinase activities and consequences on lung growth. Am J Physiol Lung Cell Mol Physiol. 2002. 282:L491–L500.

12. Moss TJ, Newnham JP, Willett KE, Kramer BW, Jobe AH, Ikegami M. Early gestational intra-amniotic endotoxin: lung function, surfactant, and morphometry. Am J Respir Crit Care Med. 2002. 165:805–811.
13. Frank L. Oxygen toxicity in neonatal rats: the effect of endotoxin treatment on survival during and post-O2 exposure. Pediatr Res. 1987. 21:109–115.

14. Cooney TP, Thurlbeck WM. The radial alveolar count method of Emery and Mithal: a reappraisal 1-postnatal lung growth. Thorax. 1982. 37:572–579.

15. Cooney TP, Thurlbeck WM. The radial alveolar count method of Emery and Mithal: a reappraisal 2-intrauterine and early postnatal lung growth. Thorax. 1982. 37:580–583.

16. Thurlbeck WM. Measurement of pulmonary emphysema. Am Rev Respir Dis. 1967. 95:752–764.
17. Ashcroft T, Simpson JM, Timbrell V. Simple method of estimating severity of pulmonary fibrosis on a numerical scale. J Clin Pathol. 1988. 41:467–470.

18. Massaro D, Teich N, Maxwell S, Massaro GD, Whitney P. Postnatal development of alveoli. Regulation and evidence for a critical period in rats. J Clin Invest. 1985. 76:1297–1305.

19. Massaro D, Massaro GD. Dexamethasone accelerates postnatal alveolar wall thinning and alters wall composition. Am J Physiol. 1986. 251:R218–R224.

20. Husain AN, Siddiqui NH, Stocker JT. Pathology of arrested acinar development in postsurfactant bronchopulmonary dysplasia. Hum Pathol. 1998. 29:710–717.

21. Bonikos DS, Bensch KG, Northway WH Jr. Oxygen toxicity in the newborn. The effect of chronic continuous 100 percent oxygen exposure on the lungs of newborn mice. Am J Pathol. 1976. 85:623–650.
22. Wilborn AM, Evers LB, Canada AT. Oxygen toxicity to the developing lung of the mouse: role of reactive oxygen species. Pediatr Res. 1996. 40:225–232.

23. Coalson JJ, Winter VT, Siler-Khodr T, Yoder BA. Neonatal chronic lung disease in extremely immature baboons. Am J Respir Crit Care Med. 1999. 160:1333–1346.

25. Moss TJ, Newnham JP, Willett KE, Kramer BW, Jobe AH, Ikegami M. Early gestational intra-amniotic endotoxin: lung function, surfactant, and morphometry. Am J Respir Crit Care Med. 2002. 165:805–811.
26. Kallapur SG, Moss TJ, Ikegami M, Jasman RL, Newnham JP, Jobe AH. Recruited inflammatory cells mediate endotoxin-induced lung maturation in preterm fetal lambs. Am J Respir Crit Care Med. 2005. 172:1315–1321.

27. Sosenko IR, Kallapur SG, Nitsos I, Moss TJ, Newnham JP, Ikegami M, et al. IL-1 alpha causes lung inflammation and maturation by direct effects on preterm fetal lamb lungs. Pediatr Res. 2006. 60:294–298.

28. Sosenko IR, Jobe AH. Intraamniotic endotoxin increases lung antioxidant enzyme activity in preterm lambs. Pediatr Res. 2003. 53:679–683.

29. Bachurski CJ, Ross GF, Ikegami M, Kramer BW, Jobe AH. Intra-amniotic endotoxin increases pulmonary surfactant proteins and induces SP-B processing in fetal sheep. Am J Physiol Lung Cell Mol Physiol. 2001. 280:L279–L285.

30. Kallapur SG, Willet KE, Jobe AH, Ikegami M, Bachurski CJ. Intra-amniotic endotoxin: chorioamnionitis precedes lung maturation in preterm lambs. Am J Physiol Lung Cell Mol Physiol. 2001. 280:L527–L536.

31. Pillow JJ, Jobe AH, Collins RA, Hantos Z, Ikegami M, Moss TJ, et al. Variability in preterm lamb lung mechanics after intra-amniotic endotoxin is associated with changes in surfactant pool size and morphometry. Am J Physiol Lung Cell Mol Physiol. 2004. 287:L992–L998.

32. Keeney SE, Mathews MJ, Shattuck KE, Dallas DV. Endotoxin protection from oxygen toxicity: effect on pulmonary neutrophils and L-selectin. Inflammation. 2002. 26:243–252.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download