Abstract
Idiopathic retroperitoneal fibrosis (IRPF) is a rare disease characterized by a retroperitoneal inflammatory proliferative fibrosing process. Hashimoto's thyroiditis is the most common inflammatory condition of the thyroid gland; and is a frequently-occurring autoimmune disorder manifesting predominantly in middle-aged women. We report a rare association of IRPF with Hashimoto's thyroiditis in a 67-year-old man demonstrating good response to steroid therapy.
Retroperitoneal fibrosis (RPF) is a rare disease characterized by development of fibrous tissue in the retroperitoneum. RPF can be classified into idiopathic and secondary forms depending on the knowledge of etiology. Secondary RPF is caused by infections, medications, surgery and malignancies, however only 30 - 40% of all cases of RPF are secondary. The etiology of most types of RPF is still unknown, and therefore they are classified as idiopathic. Because idiopathic retroperitoneal fibrosis (IRPF) shows response to immunosuppressive therapy and is frequently associated with other autoimmune diseases, it is thought to be a type of systemic autoimmune disorder. To our knowledge, only a few cases of IRPF associated with autoimmune thyroiditis such as Hashimoto's thyroiditis have been reported. We report a patient with Hashimoto's thyroiditis in whom concomitant RPF was diagnosed.
A 67-year-old man was referred for biopsy of a known retroperitoneal mass in April 2006 after suddenly developing gross hematuria two months earlier. The patient was diagnosed with Hashimoto's thyroiditis at a hospital three years previous on the basis of profound hypothyroidism, markedly increased levels of autoimmune antibody (thyroglobulin antibody 1,587.2 U/mL, normal values: 0 - 60 U/mL; microsomal antibody > 3,000 U/mL, normal values: 0 - 60 U/mL), and a diffuse hypoechogenic ultrasonographic pattern. Upon referral in April of 2006, he had been taking levothyroxine to control his Hashimoto's. Computed tomography (CT) scan of the abdomen and pelvis at a previous hospital revealed a retroperitoneal soft tissue mass extending from the lower pole of the kidney to the iliac chains, inducing bilateral ureteral obstruction. The CT did not demonstrate soft tissue between the aorta and vertebra or lymphadenopathy, excluding malignancy. A right percutaneous nephrostomy was also performed at the previous hospital in order to decompress hydronephrosis due to the retroperitoneal mass.
On admission, the patient's blood pressure was 140/100 mmHg and body temperature was 36.5℃. The lungs were clear to auscultation, heart sounds were within normal limits, and the abdomen was soft with no masses or tenderness. There was no lower extremity edema observed and the thyroid gland was not enlarged. Results of the complete blood count, electrolytes, creatinine, urea nitrogen, protein, albumin, bilirubin, β2-microglobulin and lactate dehydrogenase (LDH) were within normal ranges. Upon immunological testing, immunoglobulin G, C3 and C4 levels remained within normal ranges. No cytoplasmic antineutrophil cytoplasmic antibody or cryoglobulin was detected, and antinuclear antibodies and anti-DNA antibodies were negative. The patient showed signs of hypothyroidism with an elevated thyroid stimulating hormone (TSH) (69.56 µIU/mL, normal values: 0.4 - 3.1 µIU/mL) and reduced free T4 (0.83 ng/dL, normal values: 0.73 - 1.95 ng/dL). Upon autoantibody screening, the thyroglobulin (1,368.7 U/mL, normal values: 0 - 60 U/mL) and microsomal antibodies were elevated (> 3,000 U/mL, normal values: 0 - 60 U/mL) despite good medication compliance. After initiating an increased dose of levothyroxine (0.2 mg), laparoscopic biopsy of the retroperitoneal mass was performed.
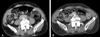
Microscopically, the mass was ill-defined and composed predominantly of chronic inflammation and fibrosis. The inflammatory cells were mainly lymphocytes and plasma cells with the occasional formation of lymphoid follicles (Fig. 1). Based on pathologic diagnosis, the patient was started on deflazacort 72 mg daily. The patient was discharged after 13 days of hospitalization and continued to receive steroid therapy at the outpatient clinic. Normal thyroid function was achieved after two months of steroid treatment; therefore levothyroxine therapy was withdrawn. Steroids were tapered to 6 mg after two months, which is the patient's current dose. The patient experienced no side effects during steroid treatment. Abdominal ultrasound demonstrated marked reduction of the retroperitoneal mass and no sign of hydronephrotic change in either kidney. Moreover, an abdominal-pelvic CT scan performed nine months after steroid treatment showed reduced mass lesion in the retroperitoneal space with resolved right side hydronephrosis (Fig. 2).
Since the first description by Ormond in 1948,1 idiopathic retroperitoneal fibrosis (IRPF) is known to be a rare disease entity characterized by a retroperitoneal inflammatory proliferative fibrosing process; which is particularly prominent around great vessels leading to ureteral obstruction.2 The incidence of IRPF is unknown and estimates vary from 1:200,000 - 1:500,000 per year.3 Hughes et al. reported that IRPF is a local immune reaction to lipid components of the atherosclerotic process in the abdominal aorta, resulting in a proximal fibrotic reaction.4 Conversely, others describe IRPF in association with various other autoimmune diseases, including primary sclerosing cholangitis, autoimmune pancreatitis, and systemic lupus erythematosus;5,6 suggesting that the pathogenesis of IRPF is closely related to an autoimmune abnormality. Because IRPF and other fibrosclerosing disorders almost exclusively have infiltrates of IgG4-positive plasma cells, a new clinicopathological entity such as IgG4-related sclerosing disease has been recently advocated.7 This suggests that IRPF is not a homogenous entity, but rather a heterogenous one.
Until now, most reports regarding the association between chronic thyroiditis and IRPF were cases of Riedel's thyroiditis. Riedel's thyroiditis is a rare disease in which the thyroid gland is replaced by fibrous tissue; and Hay et al. reported that IRPF followed Riedel's thyroiditis in 30% of cases.8 However, there have been few reports of IRPF associated with Hashimoto's thyroiditis, which is the most common inflammatory condition of the thyroid gland. So far, only five cases associating Hashimoto's thyroiditis with IRPF have been published in English literature.9-13 The mean age of all patients was 60 years, with a range from 38 to 71 years, and the female gender was predominant. Our patient was a 67-year-old man, unlike the previous reports. Including the present patient, three out of five cases showed IRPF prior to autoimmune thyroiditis, and in one case autoimmune thyroiditis appeared after retroperitoneal fibrosis. In the remaining case the two diseases occurred simultaneously, suggesting a need to monitor patients with retroperitoneal fibrosis for development of autoimmune thyroiditis.
It is difficult to distinguish Riedel's thyroiditis from the fibrosing variant of Hashimoto's thyroiditis. Nearly all patients with Hashimoto's thyroiditis have high serum concentrations of autoantibodies and profound hypothyroidism.14 Although Riedel's thyroiditis is known to show less association with autoantibody, elevated autoimmune antibody levels could be observed in 67% of Riedel's thyroiditis.15 However, most patients with Riedel's thyroiditis are euthyroid, whereas few are hypothyroid.16 Therefore, we think the present case fits the clinical picture of Hashimoto's thyroiditis. Histological confirmation would be necessary for a more accurate diagnosis; but we could not define a definite goiter or mass, making aggressive biopsy difficult.
Usual management of the disease consists of surgical relief of the ureteral obstruction, followed by corticosteroids for the prevention of recurrence.17,18 Five of the previously-reported cases used a modest dose (20 - 30 mg) of prednisolone for treatment, which resulted in optimistic results in terms of retroperitoneal fibrosis and goiter size. Moulik et al. made a report on steroid responsiveness in a case of Riedel's thyroiditis and IRPF.19 They used a modest dose of prednisolone (30 mg daily) and stopped it after 8 - 10 months, observing a decrease in the patients' thyroglobulin antibodies and thyroid peroxidase antibodies. In our case, 72 mg/day of deflazacort was initially prescribed and the dose was then decreased to 6 mg/day. Unlike previous cases which maintained 75 - 150 mcg levothyoxine continuously, we stopped levothyroxine once hypothyroidism was well controlled with deflazacort. We also observed near-disappearance of the retroperitoneal mass after steroid treatment. Since the diseases developed concurrently and both showed a positive response to steroid therapy, our case may suggest an etiologic association between the two diseases and autoimmune pathogenesis of IRPF. Recently, as mentioned above, Kimisawa et al. proposed the existence of a new entity, "IgG4-related sclerosing disease", incorporating sclerosing pancreatitis, cholangitis, and retroperitoneal fibrosis.7 This entity is characterized by extensive IgG4-positive plasma cell and T-lymphocyte infiltration of various organs. It seems that our case could be described as retroperitoneal lesions of IgG4-related systemic disease. Unfortunately, we could not confirm this possibility because testing of serum IgG4 or staining of IgG4 in tissue was not available at our hospital.
In conclusion, we have reported an unusual case of autoimmune thyroiditis with IRPF which showed good response to steroid treatment. It is important for the physician to recognize the presence of autoimmune thyroiditis in the presence of IRPF and initiate steroid therapy for resolution of both diseases prior to surgery.
Figures and Tables
References
1. Ormond JK. Bilateral ureteral obstruction due to envelopment and compression by an inflammatory retroperitoneal process. J Urol. 1948. 59:1072–1079.

2. Mitchinson MJ. The pathology of idiopathic retroperitoneal fibrosis. J Clin Pathol. 1970. 23:681–689.

3. van Bommel EF. Retroperitoneal fibrosis. Neth J Med. 2002. 60:231–242.
4. Hughes D, Buckley PJ. Idiopathic retroperitoneal fibrosis is a macrophage-rich process. Implications for its pathogenesis and treatment. Am J Surg Pathol. 1993. 17:482–490.
5. Kamisawa T, Nakajima H, Egawa N, Funata N, Tsuruta K, Okamoto A. IgG4-related sclerosing disease incorporating sclerosing pancreatitis, cholangitis, sialadenitis and retroperitoneal fibrosis with lymphadenopathy. Pancreatology. 2006. 6:132–137.

6. Okada H, Takahira S, Sugahara S, Nakamoto H, Suzuki H. Retroperitoneal fibrosis and systemic lupus erythematosus. Nephrol Dial Transplant. 1999. 14:1300–1302.
7. Kamisawa T, Okamoto A. Autoimmune pancreatitis: proposal of IgG4-related sclerosing disease. J Gastroenterol. 2006. 41:613–625.

9. Best TB, Munro RE, Burwell S, Volpé R. Riedel's thyroiditis associated with Hashimoto's thyroiditis, hypoparathyroidism, and retroperitoneal fibrosis. J Endocrinol Invest. 1991. 14:767–772.

10. Julie C, Vieillefond A, Desligneres S, Schaison G, Grunfeld JP, Franc B. Hashimoto's thyroiditis associated with Riedel's thyroiditis and retroperitoneal fibrosis. Pathol Res Pract. 1997. 193:573–577. discussion 578.

11. Armigliato M, Paolini R, Bianchini E, Monesi G, Zamboni S, D'Andrea E. Hashimoto's thyroiditis and Graves' disease associated with retroperitoneal fibrosis. Thyroid. 2002. 12:829–831.

12. Pizzini AM, Corrado S, Radighieri E, Ferretti G, Carani C, Papi G. Hashimoto's thyroiditis associated with idiopathic retroperitoneal fibrosis: case report and review of the literature. Int J Clin Pract. 2007. 61:162–164.

13. Sanai T, Hirakawa M, Yokoyama M, Soejima M, Nakayama M, Uesugi N, et al. Idiopathic retroperitoneal fibrosis associated with Hashimoto's thyroiditis: a long-term follow-up. Clin Nephrol. 2006. 66:476–478.

14. Papi G, Corrado S, Carapezzi C, De Gaetani C, Carani C. Riedel's thyroiditis and fibrous variant of Hashimoto's thyroiditis: a clinicopathological and immunohistochemical study. J Endocrinol Invest. 2003. 26:444–449.

15. Hay ID, McConahey WM, Carney JA, Woolner LB. Invasive fibrous thyroiditis (Riedel's struma) and associated extracervical fibrosclerosis: Bowlby's disease revisited [Abstract]. Ann Endocrinol (Paris). 1982. 43:29A.
16. Schwaegerle SM, Bauer TW, Esselstyn CB Jr. Riedel's thyroiditis. Am J Clin Pathol. 1988. 90:715–722.

17. Mikkelsen D, Lepor H. Innovative surgical management of idiopathic retroperitoneal fibrosis. J Urol. 1989. 141:1192–1196.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download