Abstract
Purpose
To evaluate radiological findings of non-calcified ductal carcinoma in situ (DCIS) and to correlate those with histological features.
Materials and Methods
From July 2002 to March 2006, 22 patients with histologically-proven non-calcified DCIS were included. Mammography was obtained in 19 patients, ultrasound in 18 patients, and both examinations in 15 patients. Radiological findings were evaluated according to the Breast Imaging Reporting and Data System by American College of Radiology. Histological tumor subtype and Van Nuys classification of DCIS were assessed.
Results
Histological subtypes consisted of mixed type in 11 patients (50%), comedo in 4 (18%), cribriform in 4 (18%), papillary type in 2 (9%), and solid in one (5%). According to Van Nuys classification, group 3 DCIS was observed in 13 (59%) patients. In the 19 patients who underwent mammography, 13 patients presented with abnormal findings: focal asymmetry in 7 patients (37%), masses in 4 (21%), skin thickening in one (5%), and architectural distortion in one (5%). In the 18 patients who had received breast ultrasound, a mass was present in 15 (83%) patients and ductal changes in 3 patients (17%). Sixty percent of patients with masses on ultrasound had group 3 DCIS and 100% of patients with ductal change had group 1 DCIS (p = 0.017).
For breast cancer screening, self-examination, clinical examination, and mammography have all been used for the past 20 - 30 years. Interestingly, the proportion of all breast cancers discovered because of ductal carcinoma in situ (DCIS) has increased due to the use of mammography, reaching 20 - 30% in screening groups.1-4
DCIS of the breast is defined as proliferation of malignant epithelial cells within ducts without evidence of invasion or infiltration through the basement membrane into the surrounding stroma,3 and has a much better prognosis than invasive cancers. Indeed, in a large population-based surveillance, epidemiology, and end results series, Ernster et al.5 reported a 10-year mortality risk of DCIS of only 1.9%. Therefore, early detection of DCIS is essential for improving the prognosis of breast cancer.
The typical mammographic finding of DCIS is microcalcifications, however, approximately 10 - 20% of DCIS cases manifest with non-calcified lesions.1,6,7 In addition, 16% of DCIS are occult on mammography.6,8 Thus, additional imaging modalities beside mammography are needed for detection of non-calcified DCIS.
Breast ultrasound (US) has widely been used as a supplementary modality for evaluating mammographically detected abnormalities and is an effective screening modality for detecting occult breast cancers in mammographically determined dense breasts.9-12 Indeed, US has a high sensitivity and negative predictive value for diagnosing breast cancer13 and, thanks to the added use of high resolution sonographic equipment, the characteristics of breast lesions can be depicted more clearly than ever before.
To the best of our knowledge, there have been only a few reports about the radiological findings of non-calcified DCIS, especially using breast US.14,15 Therefore, we evaluated radiological findings of non-calcified DCIS using mammography and US and correlated the radiological findings with histological features.
The institutional review board at the Korea University Anam Hospital approved this study. Using a computer database system, we searched for and recruited patients who underwent surgical treatment of primary breast cancers at our institute from July 2002 to March 2006. A total of 657 consecutive patients with primary breast carcinomas were evaluated for enrollment in this study, and 137 patients (21%) had pure DCIS on pathological examination. Among the 137 DCIS patients, 22 patients (16%) who had no microcalcifications on radiological and histological examinations were included in this study. 14 of 22 patients underwent conservation breast surgery and the remaining 8 patients underwent simple mastectomy. Sentinel lymph node biopsy was performed in 3 patients and the status of axillary nodes was negative, therefore, axillary dissection was not performed. All patients were female, ranging from 32 to 85 years of age (mean 50 years). Among the 22 patients, preoperative mammography data were available for 19 patients, US images for 18, and both for 15.
For mammography, we used a Senographe-DMR+ mammography unit (General Electronic Medical System, Milwaukee, WI, U.S.A.) or a Selenia IV (Lorad, Bedford, CT, USA). We evaluated routine craniocaudal and mediolateral oblique views, as well as additional views including magnification, spot compression, and so on in a dark room with a magnifying glass.
For US, we used a Logiq9 US unit (General Electronic Medical System) with a high frequency linear scanhead (14 - 5 MHz). We routinely scanned whole breasts and obtained transverse, longitudinal, and radial views of each lesion.
An expert breast radiologist (K.R.C) retrospectively evaluated mammography and breast US images. Mammographic findings were divided into mass, focal asymmetry, architectural distortion, or others. Other division categories included skin change, nipple change, or axillary lymphadenopathy. In cases where masses were visible on mammography, the shape, margin, and density of the masses were evaluated according to the Breast Imaging Reporting and Data System (BI-RADS®) atlas.16 US findings were divided into mass or ductal change. A mass occupies space and should be seen in two different projections. When the lesion did not exactly correspond to the definition of a mass change, but rather to echo-filled distended ducts, we defined the lesion as a ductal change. In cases where masses were found with US, the shape, margin, echo pattern, posterior acoustic features, and orientation were evaluated according to the BI-RADS®.16
An experienced pathologist (C.H.K.) evaluated the histological tumor subtype and Van Nuys classification of all 22 patients. Tumor subtypes were divided into comedo, cribriform, papillary, micropapillary, or solid type according to the guidelines set by the Consensus Conference Committee.17 Lesions with mixtures of various subtypes were defined as mixed subtypes. Van Nuys classification of DCIS was divided into group 1 (nonhigh grade DCIS without comedo-type necrosis), group 2 (nonhigh grade DCIS with comedo-type necrosis), or group 3 (high grade DCIS with or without comedo-type necrosis).18,19
The statistical analysis for correlation between radiological findings and histological features was performed by Fisher's exact test (SAS/STAT software,version 6.12; SAS Institute, Cary, NC, USA). P values lower than 0.05 were considered statistically significant.
Table 1 summarizes clinical, radiological, and histological findings of all 22 patients with non-calcified DCIS. Histological subtypes consisted of mixed type in 11 patients (50%), comedo in 4 (18%), cribriform in 4 (18%), papillary type in 2 (9%), and solid in one (5%). According to Van Nuys classification, group 3 DCIS was observed in 13 (59%) patients, group 2 in 4 (18%), and group 1 in 5 (23%). All 4 (100%) comedo subtypes and 9 (82%) of the 11 mixed subtypes displayed group 3. A total of 14 (64%) of the 22 patients had clinical symptoms: palpable masses in 11 patients and nipple discharge in 3. The 14 patients with clinical symptoms had a more frequent rate of group 3 DCIS (9/14, 64%) than patients without symptoms (4/8, 50%).
In 19 patients who received a mammography examination, 13 (68%) patients presented with abnormal findings, namely, focal asymmetry in 7 patients (37%) (Fig. 1), masses in 4 (21%), skin thickening in one (5%), and architectural distortion in one (5%) (Table 1). Of the 4 patients with masses visible on mammography, 2 patients had non-circumscribed irregular masses and the remaining 2 patients had circumscribed oval masses. We were unable to detect abnormalities in the remaining 6 (32%) patients, 5 of which had dense breast tissues (Fig. 2) while the other had scattered fibroglandular tissues (Fig. 3). Among these 6 patients, 2 (33%) had group 3 DCIS on histological examination. Four (57%) of 7 patients with focal asymmetry on mammography had group 3 DCIS, while 3 (75%) of 4 patients with masses had group 3 DCIS. However, there was no statistical significance between mammographic findings and Van Nuys classification (p = 0.811).
In 18 patients who had received breast US, a mass change was present in 15 (83%) patients (Figs. 1 and 2) and ductal changes in 3 (17%) remaining patients (Table 1) (Fig. 3). Nine (60%) of 15 patients with masses on breast US had group 3 DCIS. Conversely, all 3 patients with ductal change on US had group 1 cribriform DCIS. A mass was more frequently associated with group 3 DCIS, and ductal change was with group 1 DCIS (p = 0.017). Table 2 presents the characteristics of 15 masses evaluated with breast US, and Table 3 demonstrates a correlation between US findings and Van Nuys classification of these masses. Non-circumscribed margins (11/15, 73%) including indistinct, microlobulated, or spiculated margins were more frequent than circumscribed margins (4/15, 27%). Indistinct margins were observed in 8 patients (44%); this was the most common margin observed in non-calcified DCIS. Group 3 DCIS was more common in masses with non-circumscribed margins (9/11, 82%) than masses with circumscribed margins (1/4, 25%). Most of the DCIS cases had a hypoechoic or complex echo pattern (12/15, 80%) and parallel orientation (13/15, 87%). The two non-parallel oriented masses were group 3 DCIS. The shapes and posterior acoustic features of non-calcified DCIS varied.
DCIS is mostly detected during screening mammography, because clinical symptoms are present in only 10 - 24% of DCIS patients.19 While the most common feature of mammography-detected DCIS is microcalcification, the focus of our present study was on non-calcified DCIS. Ikeda and Andersson6 reported that 60 (82%) out of 73 patients with non-calcified DCIS are symptomatic. In agreement with the about results of Ikeda and Andersson, we found that 64% of non-calcified DCIS patients were symptomatic: 50% palpable masses and 14% nipple discharge. In our clinical practice, we routinely performed breast US with or without mammography if a patient had a breast palpable mass. If the mass had suspicious malignant feature on physical examination, a physician tried to perform fine needle aspiration or core biopsy. When the cytological result after fine needle aspiration was inadequate to decide histological diagnosis, imaging-guided core needle biopsy or excisional biopsy was performed. When the histological result after core biopsy was high risk breast lesions (i.e. atypical ductal hyperplasia or radial scar) or DCIS, we performed surgical excision for final histological diagnosis. In cases with nipple discharge, we performed both mammography and breast US. Galactography or breast MRI would be considered, if there was no abnormal finding on mammography and US. In 22 patients of the current study, initial histological diagnoses in 17 patients were obtained by core needle biopsy, excisional biopsy in 4 patients, and fine-needle aspiration in one patient and then surgery was performed in all 22 patients. Sixty four percent of non-calcified DCIS patients were symptomatic in this study, therefore, physician should evaluate the patients who have clinical symptoms with more attention.
Histological nuclear grades and presence of comedo necrosis in DCIS are important for predicting a prognosis. Van Nuys classification was made using both nuclear grade and presence of comedo necrosis, and DCIS cases were divided into group 1, 2 or 3.18,19 Group 3 DCIS has both high nuclear grade and comedo necrosis. In calcified DCIS, linear branching or pleomorphic microcalcifications have a high predictive value for the high-grade comedo type.20 On the other hand, there have been only a few reports showing the histological feature in non-calcified DCIS, the results of which are controversial. In the present study we found that 13 (59%) of 22 patients had group 3 DCIS. With respect to tumor subtype, mixed type was the most common (50%) in non-calcified DCIS, whereas comedo type (18%) and cribriform type (18%) were the second most common non-calcified DCIS. However, the number of patients enrolled in many previous studies on this topic has been too small to analyze statistical significance. Therefore, further study is needed to confirm characteristic histological feature of non-calcified DCIS in a large population
In this study, we evaluated radiological findings using both mammography and US. At 37%, focal asymmetries were the most common finding on mammography, followed by masses at 21%, and no lesions at 32%. A focal asymmetry differs from a mass since it usually lacks convex outward borders and contains interspersed fat.16 Thus, radiologists should examine mammography in detail and distinguish a focal asymmetric density from asymmetric breast tissue. If such a lesion is suspected, physicians should not hesitate to perform a biopsy.
In the present study, 6 (32%) of the non-calcified DCIS cases could not be detected on mammography. Five (83%) out of six DCIS patients with no mammographical lesions had dense breast tissues on mammography, while the other patient exhibited scattered fibroglandular tissues. Previous studies demonstrated that 6 - 23% of overall DCIS cases were mammographically occult.6-8 These 6 patients presented five masses and one instance of ductal change according to breast US. For this reason, breast US was the only modality able to detect the lesion. Thus, breast US should be considered as a screening modality for the detection of non-calcified DCIS in women with dense breast tissues on mammography.
In the current study, 15 (83%) of 18 patients who received breast US presented with masses, while 3 patients presented with ductal change (17%). Common findings of the masses included an indistinct margin, hypoechoic or complex echoic patterns, and a parallel orientation. Our US findings were similar to previous studies by Moon et al.15 and DiPiro et al.14 who evaluated overall DCIS cases, calcified and non-calcified. Typical malignant features, including spiculation or shadowing, were not frequent in DCIS. Indistinct margins and hypoechoic or complex echo patterns are suspicious findings in the diagnosis of breast malignancy; however, round or oval shape and unaffected posterior acoustic features are not. Thus, if a mass has one more suspicious malignant findings by breast US, a biopsy should be performed in order to diagnose possible non-calcified DCIS.
There have been several studies outlining the ductal changes that occur in DCIS. Moon et al.15 demonstrated that multiple small intraductal nodules are rare presentation of DCIS, especially in patients with nipple discharge, and Yang and Tse21 reported that 23% of symptomatic DCIS can be depicted as ductal dilatations. In the present study, patients with ductal changes presented single or multiple tumor-filled ductal dilatations on US. On histological examination, there were intraductal and/or periductal tumor cell infiltrations with minor periductal fibrosis. According to the current BI-RADS-Ultrasound lexicon, ductal change is defined as an abnormal caliber and/or arborization; there is no mention of intraductal nodules. Therefore, we hope that the findings in this study will prompt to reevaluate the description of ductal change in the current BI-RADS-Ultrasound lexicon.
In conclusion, most non-calcified DCIS patients have clinical symptoms and group 3 DCIS of Van Nuys classification. Such cases commonly present a focal asymmetry on mammography, and occult cases are not uncommon. High resolution US can be useful for detecting non-calcified DCIS when the lesion cannot fully be assessed by mammography. In addition, US findings are correlated with histological features. However, the number of patients was too small to statistically analyze. Thus, further studies are needed to confirm the correlation between US findings and histological features in patients with non-calcified DCIS.
Figures and Tables
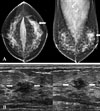 | Fig. 1(Patient No. 5) A 35-year-old woman with a palpable mass in left breast and group 3 DCIS of Van Nuys classification (A) Mammography shows a focal asymmetry (arrows) in the left upper outer quadrant, palpable mass site. (B) Transverse (left) and longitudinal (right) scans of US reveal a microlobulated marginated, irregular shaped, hypoechoic mass (arrows). The mass has parallel orientation and an unaffected posterior acoustic feature. |
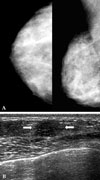 | Fig. 2(Patient No. 8) A 42 -year-old woman with a palpable mass in the right breast and group 3 DCIS of Van Nuys classification. (A) Right mammography shows no detectable lesion. (B) Transverse scan of US demonstrates an indistinct marginated, oval shaped, hypoechoic mass (arrows) in the lower mid portion of right breast, palpable mass site. The mass has parallel orientation and an unaffected posterior acoustic feature. |
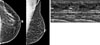 | Fig. 3(Patient No. 12) A 45-year-old woman with left bloody discharge and group 1 DCIS of Van Nuys classification. (A) Mammography shows no detectable abnormal finding. (B) Transverse scan of US depicts ductectasia with intraductal solid components (arrows) in the central portion of the left breast. |
References
1. Dershaw DD, Abramson A, Kinne DW. Ductal carcinoma in situ: mammographic findings and clinical implications. Radiology. 1989. 170:411–415.

2. Holland R, Hendriks JH, Vebeek AL, Mravunac M, Schuurmans Stekhoven JH. Extent, distribution, and mammographic/histological correlations of breast ductal carcinoma in situ. Lancet. 1990. 335:519–522.

3. Schnitt SJ, Silen W, Sadowsky NL, Connolly JL, Harris JR. Ductal carcinoma in situ (intraductal carcinoma) of the breast. N Engl J Med. 1988. 318:898–903.

4. Stomper PC, Connolly JL. Ductal carcinoma in situ of the breast: correlation between mammographic calcification and tumor subtype. AJR Am J Roentgenol. 1992. 159:483–485.

5. Ernster VL, Barclay J, Kerlikowske K, Wilkie H, Ballard-Barbash R. Mortality among women with ductal carcinoma in situ of the breast in the population-based surveillance, epidemiology and end results program. Arch Intern Med. 2000. 160:953–958.

6. Ikeda DM, Andersson I. Ductal carcinoma in situ: atypical mammographic appearances. Radiology. 1989. 172:661–666.

7. Stomper PC, Connolly JL, Meyer JE, Harris JR. Clinically occult ductal carcinoma in situ detected with mammography: analysis of 100 cases with radiologic-pathologic correlation. Radiology. 1989. 172:235–241.

8. Holland R, Peterse JL, Millis RR, Eusebi V, Faverly D, van de Vijver MJ, et al. Ductal carcinoma in situ: a proposal for a new classification. Semin Diagn Pathol. 1994. 11:167–180.
9. Berg WA, Gilbreath PL. Multicentric and multifocal cancer: whole-breast US in preoperative evaluation. Radiology. 2000. 214:59–66.

10. Crystal P, Strano SD, Shcharynski S, Koretz MJ. Using sonography to screen women with mammographically dense breasts. AJR Am J Roentgenol. 2003. 181:177–182.

11. Kaplan SS. Clinical utility of bilateral whole-breast US in the evaluation of women with dense breast tissue. Radiology. 2001. 221:641–649.

12. Leconte I, Feger C, Galant C, Berlière M, Berg BV, D'Hoore W, et al. Mammography and subsequent whole-breast sonography of nonpalpable breast cancers: the importance of radiologic breast density. AJR Am J Roentgenol. 2003. 180:1675–1679.

13. Stavros AT, Thickman D, Rapp CL, Dennis MA, Parker SH, Sisney GA. Solid breast nodules: use of sonography to distinguish between benign and malignant lesions. Radiology. 1995. 196:123–134.

14. DiPiro PJ, Meyer JE, Denison CM, Frenna TH, Harvey SC, Smith DN. Image-guided core breast biopsy of ductal carcinoma in situ presenting as a non-calcified abnormality. Eur J Radiol. 1999. 30:231–236.

15. Moon WK, Myung JS, Lee YJ, Park IA, Noh DY, Im JG. US of ductal carcinoma in situ. Radiographics. 2002. 22:269–280. discussion 80-81.

16. American College of Radiology. BI-RADS Committee. ACR BI-RADS® breast imaging and reporting data system: breast imaging atlas. 2003. Reston, VA: American College of Radiology.
17. Consensus Conference Committee. Consensus conference on the classification of ductal carcinoma in situ. Hum Pathol. 1997. 28:1221–1225.
18. Poller DN, Silverstein MJ, Galea M, Locker AP, Elston CW, Blamey RW, et al. Ideas in pathology. Ductal carcinoma in situ of the breast: a proposal for a new simplified histological classification association between cellular proliferation and c-erbB-2 protein expression. Mod Pathol. 1994. 7:257–262.
19. Silverstein MJ, Poller DN, Waisman JR, Colburn WJ, Barth A, Gierson ED, et al. Prognostic classification of breast ductal carcinoma-in-situ. Lancet. 1995. 345:1154–1157.

20. Holland R, Hendriks JH. Microcalcifications associated with ductal carcinoma in situ: mammographic-pathologic correlation. Semin Diagn Pathol. 1994. 11:181–192.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download