Abstract
Purpose
Effect of recombinant human growth hormone (rhGH) administration on lipid storage, and its subsequent effect on insulin sensitivity have not yet been adequately examined. Thus, we investigated the effects of rhGH treatment on muscle triglyceride (TG) and ceramide content, and insulin sensitivity after 4 weeks of rhGH administration in rats.
Materials and Methods
Fourteen rats were randomly assigned to two groups: rhGH injection group (GH, n = 7) and saline injection group (CON, n = 7). GH received rhGH by subcutaneous injections (130 µg·kg-1·day-1, 6 days·week-1) for 4 weeks, while CON received saline injections that were equivalent in volume to GH group. Intramuscular TG and ceramide content and hepatic TG content were measured. To determine insulin sesitivity, oral glucose tolerance test (OGTT) and muscle incubation for glucose transport rate were performed in rats, and used as indicators of insulin sensitivity. We also examined plasm lipid profiles.
Results
After 4 weeks of rhGH treatment, the GH group had higher muscle and liver TG contents than the CON (p < 0.05). Ceramide content in GH was significantly greater than that in CON (p < 0.05). GH also had higher plasma levels of FFA (p < 0.05), glucose and insulin responses during OGTT (p < 0.05), and lower glucose transport rates in submaximal insulin concentration (p < 0.05) as compared with CON. Results indicate that rhGH treatment is associated with insulin resistance in rats.
The recombinant human growth hormone (rhGH) has been developed for adults with growth hormone deficiency (GHD), and its physiological and therapeutic effects are well documented in the literature.1-3 Notably, administration of rhGH is reported to antagonize the effects of insulin on lipid and glucose metabolism;4-9 in vivo studies demonstrate that rhGH administration can reduce insulin and glucose responses in rats.5,8,10,11
The mechanism responsible for the observed association between rhGH and insulin resistance is not well understood, but increased levels of free fatty acid (FFA) and reduced levels of GLUT-4 protein molecules have been implicated.12-17 Another plausible mechanism for insulin resistance is that elevated FFA levels can enhance the accumulation of intramuscular triglyceride (TG) content, which may contribute to insulin resistance in skeletal muscle. Some investigators have shown an inverse association between intramuscular TG content and insulin sensitivity.18,19 However, little is known about this association after rhGH treatment. Therefore, we investigated the effects of chronic in vitro rhGH administration and hypothesized that it will increase intramuscular TG content and reduce insulin sensitivity. The results from this study support that hypothesis.
Fourteen 4-week-old Sprague-Dawley male rats (Samtaco Bio Korea, Inc; body weight 128.5 ± 3 g) were housed one per cage in a 12 : 12 light-dark cycle at 24℃, and were given Harlan rat chow (Harlan Teklad) with tap water ad libitum. After 7 days of adaptation to the laboratory environment, the rats were randomly assigned to two groups: 1) saline injection group (CON, n = 7) and 2) rhGH injection group (GH, n = 7). The GH group received rhGH (Eutropin®, LG inc, Gyeonggi, Korea) by subcutaneous injections (130 µg·kg-1·day-1, 6 days·week-1) for 4 weeks, while the CON group received saline injections as equivalent volume as the GH group. All protocols were approved by the Kyungpook National University Animal Care and Use Committee.
After 4 weeks of treatment and following a 12 hours fast, the rats were given 50% aqueous glucose solution (1 g·kg-1 body weight) using a stomach tube. The rats were placed in acrylic restrainers on a heating pad, and approximately 0.5 mL of blood was taken from the tail immediately prior to glucose administration, and at 30 and 60 minutes after administration. To determine plasma insulin levels, blood samples were placed in microcentrifuge tubes (Cole-parmer international, Vernon hills, IL, USA) containing 30 µL Heparin (Choongwae Pharma. Co., Jeonbuk, Korea), and centrifuged at 10,000 g for 20 minutes. The plasma portion was removed and stored at - 80℃ until further analysis. Insulin resistance was determined under submaximal insulin concentrations during muscle incubation.
One week after the OGTT, the rats were fasted for 8 hours and then anesthetized by intraperitoneal injection with sodium pentobarbital (6.5 mg·kg-1). The epitrochlearis muscles were surgically isolated and removed to assess glucose transport rate in muscle,20 while the liver and plantaris muscle were excised to assay the TG content. Blood samples were drawn from an abdominal aorta to measure plasma glucose, insulin, total cholesterol, triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), and FFA levels.
Glucose transport activity was measured using 3-MG (3-O-[3H]-methylglucose). After dissection, muscles were rinsed briefly in 25-mL flasks containing 3 mL of KHB (Krebs-Henseleit buffer, 0.1% BSA) and then transferred to recovery vials with a recovery medium (32 mM mannitol, 8 mM glucose). Following this 30 minutes recovery period, the muscles were transferred to pre-incubation vials, containing KHB as a preincubation medium (8 mM glucose, 32 mM mannitol, and 0.1% BSA), and then incubated for 20 minutes. After 20 minutes of pre-incubation, the muscles were rinsed with fresh KHB medium-supplement (40 mM mannitol) and transferred to final incubation vials. The final incubation medium contained 3-O-[3H]-methylglucose (2.2 µCi·mmol-1) and [14C] mannitol (0.2 µCi·mmol-1) in the presence or absence of a submaximal (1,000 µIU·mL-1) dose of insulin. Throughout the incubation process, samples were maintained at 35℃ during the recovery and pre-incubation periods; at 30℃ during the rinse; at final incubation in a shaking water bath (120 cycles·min-1); and at gassed with a 95% O2 and 5% CO2 mixture. The muscles were then blotted, clamp-frozen, and processed for determination of intracellular 3-MG accumulation and extracellular space. To determine glucose transport rate, muscle samples were analyzed in a liquid scintillation counter (Beckman Instruments, Inc, Fullerton, CA, USA) by setting the channels at simultaneous 3H and 14C readings.21
Portions of gasrtrocnemius muscle were homogenized for 15 seconds in ice-cold HES buffer (20 mM HEPES, 1 mM EDTA, and 250 mM sucrose), using a motor driven homogenizer (Art-Miccra D-8 Model, Art Labortechnik, Müllhein, Germany). Sample homogenates and standards were diluted by 1 : 2 with 2x Laemmli sample buffer (S3401, Sigma-Aldrich, St. Louis, MO, USA) and incubated for 20 minutes. Muscle homogenates, which contained 50 µg of protein, were then subjected to SDS-polyacrylamide-gel-electrophoresis under reducing conditions of a 10% resolving gel. Resolved proteins were transferred to a nitrocellulose membrane (BioRad, Hercules, CA, USA) and blocked for 60 minutes with 5% non-fat milk. Membranes were incubated with GLUT-4 antiserum (Santa Cruz Biotechology, Santa cruz, CA, USA), diluted with 1 : 10,000 in a T-TBS/5% dry milk, for 90 minutes. Membrane were washed with T-TBS and incubated with secondary antibody (ZYMED Labolatories; at a dilution of 1 : 5000 with T-TBS/1% non-fat milk) for 60 minutes at room temperature, and washed with T-TBS. The GLUT-4 protein was visualized by Hyperfilm (Eastman Kodak, Rochester, NY, USA) using the Western blot luminal reagent (Santa Cruz Biotechology, Santa cruz, CA, USA).
Plantaris muscles were homogenized in buffer (0.25 M sucrose, 25 mM KC1, 50 mM Tris, 0.5 mM EDTA; pH7.4). After homogenization, lipid was extracted by adding a chloroform, methanol, and buffer water mixture maintained at the ratio of 0.5:1.0:0.4 and 1.0:1.0:0.9 (v/v/v) before and after dilution, respectively. Lipid extraction and ceramide determination were carried out according to the guidelines described by Bielawaka et al.22
Liver samples were homogenized in 50 mM potassium phosphate and 1 mM EDTA (pH 7.4) with 1 : 15 dilution, while plantaris muscle samples were homogenized as 1 : 20 dilution. Lipid was determined by following guidelines described by Burton and Anderson.23 Homongenized samples were centrifuged at 1,000 g for 10 minutes, and the supernatant was removed by adding 0.5 mL of 2-propanol to prevent any evaporation of TG. The TG content was measured using an enzymatic method (Elitech, Sees, France) and quantified in the skeletal muscle of both groups.
Plasma glucose concentrations were determined by a glucose analyzer (Model YSI-23A, Yellow Springs Instruments, Yellow Springs, OH, USA). Plasma insulin and leptin concentrations were measured via radioimmunoassay (Linco Research Inc., St. Louis, MO, USA) by using a double antibody procedure.24 Plasma TC, TG, and HDL-C levels were measured by an enzymatic method (Elitech, Sees, France). Plasma FFA was determined by an acyl-CoA oxidase-based colorimetric kit.25
Independent t tests were used to compare mean differences for FFA levels, GLUT-4 contents, TG contents, and glucose transport rates between the GH and CON groups. Two-way ANOVA with repeated measures were used to determine plasma glucose and insulin responses across group by time factor. The sphericity assumption was justified using Hyunh-Feldt Epsilon (ε) test. Pearson correlation with simple regression was used to investigate the association between TG content and glucose transport rate. All statistical procedures were performed by SPSS (ver. 14) with a significance level at 0.05.
There was no statistical difference in growth rate between the GH and CON groups (Fig. 1).
Biochemical characteristics after 4 weeks of rhGH treatments are presented in Table 1. The GH group (0.83 ± 0.07 mmol·L-1) had higher FFA values than the CON group did (0.58 ± 0.05 mmol·L-1, p < 0.05). There were no statistical differences in TC, TG, and HDL-C values between the 2 groups.
As shown in Fig. 2, the GH group had higher glucose levels than the CON group at basal time (GH 132.6 ± 5.0 vs CON 98.9 ± 4.5 mg·dL-1, p < 0.05) and at 60 minutes (GH 182.0 ± 13.2 vs CON 148.7.90 ± 6.1 mg·dL-1, p < 0.05) as compared with the CON group, respectively. The GH group also had higher insulin levels at basal time (GH 1.95 ± 0.36 vs CON 0.98 ± 0.07 µU·mL-1, p < 0.05), at 30 minutes (GH 4.53 ± 0.42 vs CON 2.54 ± 0.35 µU·mL-1, p < 0.05), and at 60 minutes (GH 3.62 ± 0.21 vs CON 1.81 ± 0.21 µU·mL-1, p < 0.05) as compared with the CON group, respectively.
There was no statistical difference in GLUT-4 contents between the GH (RG: 37.7 ± 2.0%, WG: 21.3 ± 1.5%) and CON (RG: 36.6 ± 2.3%, WG: 22.3 ± 1.6%) groups (Fig. 3).
Fig. 4 shows that the GH group (1.56 ± 0.12 µmol·g-1) had greater TG content in the plantaris muscle compared with the CON group (0.79 ± 0.08 µmol·g-1, p < 0.05). The GH group also had significantly higher TG content in the liver (9.29 ± 1.46 µmol·g-1) than with the CON group (5.12 ± 0.59 µmol·g-1, p < 0.05).
Fig. 5 shows that the GH group (83.8 ± 2.1 pmol·mg-1) had greater ceramide content in the skeletal muscle than with the CON group (75.7 ± 2.7 pmol·mg-1, p < 0.05).
As shown in Fig. 6, the GH group had a lower glucose transport rate under submaximal insulin concentrations (2.36 ± 0.18 µmol·mL-1·hr-1) than the CON group (3.26 ± 0.26 µmoL·mL-1·hr-1, p < 0.05). However, there was no statistical difference between these 2 groups under non-insulin conditions.
Fig. 7 shows the association between glucose transport rate and muscle TG content. There was a significant inverse association between muscle TG content and glucose transport rate (r = - 0.67, p < 0.01).
In the present study, we observed a decrease in plasma glucose and insulin responses during OGTT and glucose transport rate during muscle incubation under submaximal insulin concentration in rhGH injected rats (130 µg·kg-1·day-1 for 4 weeks). Also, we observerd that prolonged exposure to rhGH elevated intramuscular TG and ceramide levels in rats. In addition, plasma FFA and hepatic TG content were also elevated when rhGH was administered. Thus, the major finding of this study is that the decreased insulin sensitivity due to chronic rhGH administration would partly result from an elevated intramuscular ceramide content which is deriveded from the elevated muscle TG content.
It has been reported that there was an inverse relationship between insulin sensitivity and intramuscular TG stroage,19,26 similar to the results of the present study. However, elevated intramuscular TG storage in itself did not reduce muscle insulin sensitivity, but rather acts as a source for lipid derivatives, such as diacylgycerol, long chain fatty acid acyl-CoA or ceramides, that directly affect insulin signalling and, thus, insulin sensitivity.27-29 In previous studies,27 these was a possibility that ceramide might inhibit insulin signalling at the level of protein kinase B (PKB)/Akt.30 Therefore, we hypothesized that the decreased glucose transport due to rhGH injection might partly result from elevations of intramuscular TG storage and ceramide content. In the current investigation, we observed that rhGH administation significantly elevated muscle TG storage and ceramide content. Thus, intramuscular ceramide elevation due to rhGH injection may partly contribute to the development of insulin resistance
It has also been postulated that FFA levels due to rhGH treatment are responsible for insulin resistance.31,32 The elevated FFA levels demonstrated here is consistent with previous reports.16,33 Many previous studies reported that an increase in circulating FFA is a key factor for the development of insulin resistance in insulin sensitive tissues such as adipocytes and skeletal muscle.13,34,35 Increased level of FFA by rhGH treatment has been suggested to be related to insulin resistance.14,16,36 In vitro studies have indicated that the lipolytic actions of rhGH may involve stimulation of gene expression after binding to the rhGH receptor and subsequent activation of JAK2 tyrosine kinase, adenyl cyclase, and stimulation of cAMP production, triggering the hormone-sensitive lipase by increasing circulating FFA.37-39 According to the glucose-FFA cycle postulated by Randle et al.,40 increased FFA concentrations may decrease the uptake of glucose in skeletal muscle. Skeletal muscle is responsible for 70 - 80% of whole body insulin-stimulated glucose uptake and is, therefore, considered to be the most important site of insulin resistance.13 The mechanism by which raised plasma FFA concentrations inhibit insulinstimulated glucose uptake in muscle may involve the insulin-signaling pathway responsible for GLUT4 translocation, including IRS-1 phosphorylation, PI3K activity, or glycogen synthase activity.41-44 In addition, an inverse correlation between muscle lipid content and insulin sensitivity has been demonstrated in humans by muscle biopsy,19 computed tomography45 and magnetic resonance spectroscopy.26 TG stored within the muscle fiber has been implicated as a casual factor in the insulin resistance-FFA relationship, and the postulated mechanism involves intramyocellular accumulation of diacylglycerol, activation of protein kinase C, or myoplasmic long-chain fatty acyl CoA.46,47
We also observed that GLUT-4 protein levels were similar between the GH and CON groups. GLUT-4 is the major glucose transporter protein in skeletal muscle, and the muscle GLUT-4 protein levels are significantly related to the rate of glucose transport during muscle incubation.48 Therefore, any decrease in the rate of glucose transport in rhGH-treated rats would seem to require a reduction of GLUT-4 levels in skeletal muscle. However, our data showed no reduction of muscle GLUT-4 protein levels in the rhGH-treated rats, and suggest that the deflect in the signalling mechanism occurred prior to the glucose translocation process. This result is consistent with the previous reports that muscle GLUT-4 protein levels were not altered in rhGH-treated rats,10,41 and supports the concept that alterations of muscle GLUT-4 protein levels are not responsible for the reduction of insulin-stimulated glucose disposal in rhGH-treated rats.
It is plausible that rhGH-treated rats develop insulin resistance in skeletal muscle due to elevated intramuscular TG storage and ceramide content. Elevation of circulating FFA levels due to rhGH treatment enhances intramuscular TG content, and high levels of muscle TG and ceramide content are responsible for insulin sensitivity in skeletal muscle. Our data also showed that reduced glucose transport rate in the rhGH-treated rats occurred with no change in GLUT-4 content, and was directly related to the TG content of the muscle. A strength of this study is that, to our knowledge, this is the first study to specifically investigate the association between muscle TG content and glucose transport rate after the rhGH treatment. Our study is also well designed to eliminate all possible confounding factors using a randomized control design. Further studies are needed to validate our findings across different murines as well as in humans.
In conclusion, we found that rhGH treatment is directly associated with insulin resistance in rats. The causative factor for insulin resistance is that rhGH treatment elevates TG content and ceramide in muscle, and an elevated TG and ceramide contents are inversely associated with insulin sensitivity in rats.
Figures and Tables
 | Fig. 2Effect of growth hormone administration on plasma glucose (A) and insulin (B) concentrations during OGTT. CON, control group; GH, growth hormone group; OGTT, oral glucose tolerance test. Each point represents the mean ± SE.
*p < 0.05.
|
 | Fig. 3GLUT-4 contents in red garstrocnemius (RG) (A) and white garstrocnemius (WG) (B) muscle. CON, control group; GH, growth hormone group. Each point represents the mean ± SE. |
 | Fig. 4Triglyceride contents in plantaris muscle (A) and liver (B). CON, control group; GH, growth hormone group. Each point represents the mean ± SE. *p < 0.05. |
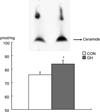 | Fig. 5Ceramide contents in garstrocnemius muscle . CON, control group; GH, growth hormone group. Each point represents the mean ± SE. *p < 0.05. |
 | Fig. 6Glucose transport rate in epitrochlearis muscle with non- (B) and submaximal insulin conditions (A). CON, control group; GH, growth hormone group. Each point represents the mean ± SE. *p < 0.05. |
References
1. Doga M, Bonadonna S, Gola M, Mazziotti G, Giustina A. Growth hormone deficiency in the adult. Pituitary. 2006. 9:305–311.

2. Henwood MJ, Grimberg A, Moshang T Jr. Expanded spectrum of recombinant human growth hormone therapy. Curr Opin Pediatr. 2002. 14:437–442.

3. Woodhouse LJ, Mukherjee A, Shalet SM, Ezzat S. The influence of growth hormone status on physical impairments, functional limitations, and health-related quality of life in adults. Endocr Rev. 2006. 27:287–317.

4. Bramnert M, Segerlantz M, Laurila E, Daugaard JR, Manhem P, Groop L. Growth hormone replacement therapy induces insulin resistance by activating the glucose-fatty acid cycle. J Clin Endocrinol Metab. 2003. 88:1455–1463.

5. Cartee GD, Bohn EE. Growth hormone reduces glucose transport but not GLUT-1 or GLUT-4 in adult and old rats. Am J Physiol. 1995. 268(5 Pt 1):E902–E909.

6. Chrisoulidou A, Beshyah SA, Rutherford O, Spinks TJ, Mayet J, Kyd P, et al. Effects of 7 years of growth hormone replacement therapy in hypopituitary adults. J Clin Endocrinol Metab. 2000. 85:3762–3769.

7. Groop L, Segerlantz M, Bramnert M. Insulin sensitivity in adults with growth hormone deficiency and effect of growth hormone treatment. Horm Res. 2005. 64 Suppl 3:45–50.

8. Ng SF, Storlien LH, Kraegen EW, Stuart MC, Chapman GE, Lazarus L. Effect of biosynthetic human growth hormone on insulin action in individual tissues of the rat in vivo. Metabolism. 1990. 39:264–268.

9. Yuen KC, Dunger DB. Impact of treatment with recombinant human GH and IGF-I on visceral adipose tissue and glucose homeostasis in adults. Growth Horm IGF Res. 2006. 16 Suppl A:S55–S61.

10. Hou CW, Chou SW, Ho HY, Lee WC, Lin CH, Kuo CH. Interactive effect of exercise training and growth hormone administration on glucose tolerance and muscle GLUT4 protein expression in rats. J Biomed Sci. 2003. 10(6 Pt 2):689–696.

11. Riddick FA Jr, Reisler DM, Kipnis DM. The sugar transport system in striated muscle. Effect of growth hormone, hydrocortisone and alloxan diabetes. Diabetes. 1962. 11:171–178.
12. Binnerts A, Swart GR, Wilson JH, Hoogerbrugge N, Pols HA, Birkenhager JC, et al. The effect of growth hormone administration in growth hormone deficient adults on bone, protein, carbohydrate and lipid homeostasis, as well as on body composition. Clin Endocrinol (Oxf). 1992. 37:79–87.

13. Ivy JL, Zderic TW, Fogt DL. Prevention and treatment of non-insulin-dependent diabetes mellitus. Exerc Sport Sci Rev. 1999. 27:1–35.
14. Jørgensen JO, Krag M, Jessen N, Nørrelund H, Vestergaard ET, Moller N, et al. Growth hormone and glucose homeostasis. Horm Res. 2004. 62 Suppl 3:51–55.

15. Kim JK, Choi CS, Youn JH. Acute effect of growth hormone to induce peripheral insulin resistance is independent of FFA and insulin levels in rats. Am J Physiol. 1999. 277(4 Pt 1):E742–E749.

16. Møller N, Gjedsted J, Gormsen L, Fuglsang J, Djurhuus C. Effects of growth hormone on lipid metabolism in humans. Growth Horm IGF Res. 2003. 13 Suppl A:S18–S21.

17. Zisman A, Peroni OD, Abel ED, Michael MD, Mauvais-Jarvis F, Lowell BB, et al. Targeted disruption of the glucose transporter 4 selectively in muscle causes insulin resistance and glucose intolerance. Nat Med. 2000. 6:924–928.

18. Hegarty BD, Cooney GJ, Kraegen EW, Furler SM. Increased efficiency of fatty acid uptake contributes to lipid accumulation in skeletal muscle of high fat-fed insulin-resistant rats. Diabetes. 2002. 51:1477–1484.

19. Pan DA, Lillioja S, Kriketos AD, Milner MR, Baur LA, Bogardus C, et al. Skeletal muscle triglyceride levels are inversely related to insulin action. Diabetes. 1997. 46:983–988.

20. Kawano Y, Rincon J, Soler A, Ryder JW, Nolte LA, Zierath JR, et al. Changes in glucose transport and protein kinase Cbeta(2) in rat skeletal muscle induced by hyperglycaemia. Diabetologia. 1999. 42:1071–1079.

21. Young JC, Balon TW. Role of dihydropyridine sensitive calcium channels in glucose transport in skeletal muscle. Life Sci. 1997. 61:335–342.

22. Bielawska A, Perry DK, Hannun YA. Determination of ceramides and diglycerides by the diglyceride kinase assay. Anal Biochem. 2001. 298:141–150.

23. Burton AF, Anderson FH. Increased cholesteryl ester content in liver of mice fed lipid emulsion diets high in polyunsaturated fats. JPEN J Parenter Enteral Nutr. 1985. 9:480–482.

24. Morgan CR, Lazarow A. Immunoassay of insulin using a two-antibody system. Proc Soc Exp Biol Med. 1962. 110:29–32.

25. Noma A, Okabe H, Kita M. [Determination of serum cholinesterase activity by means of automatic titration]. Rinsho Byori. 1973. 21:457–460.
26. Virkamäki A, Korsheninnikova E, Seppälä-Lindroos A, Vehkavaara S, Goto T, Halavaara J, et al. Intramyocellular lipid is associated with resistance to in vivo insulin actions on glucose uptake, antilipolysis, and early insulin signaling pathways in human skeletal muscle. Diabetes. 2001. 50:2337–2343.
28. Summers SA, Garza LA, Zhou H, Birnbaum MJ. Regulation of insulin-stimulated glucose transporter GLUT4 translocation and Akt kinase activity by ceramide. Mol Cell Biol. 1998. 18:5457–5464.

29. Straczkowski M, Kowalska I, Baranowski M, Nikolajuk A, Otziomek E, Zabielski P, et al. Increased skeletal muscle ceramide level in men at risk of developing type 2 diabetes. Diabetologia. 2007. 50:2366–2373.

30. Schmitz-Peiffer C, Craig DL, Biden TJ. Ceramide generation is sufficient to account for the inhibition of the insulin-stimulated PKB pathway in C2C12 skeletal muscle cells pretreated with palmitate. J Biol Chem. 1999. 274:24202–24210.

31. Krag MB, Gormsen LC, Guo Z, Christiansen JS, Jensen MD, Nielsen S, et al. Growth hormone-induced insulin resistance is associated with increased intramyocellular triglyceride content but unaltered VLDL-triglyceride kinetics. Am J Physiol Endocrinol Metab. 2007. 292:E920–E927.

32. Segerlantz M, Bramnert M, Manhem P, Laurila E, Groop LC. Inhibition of lipolysis during acute GH exposure increases insulin sensitivity in previously untreated GH-deficient adults. Eur J Endocrinol. 2003. 149:511–519.

33. Adiels M, Taskinen MR, Packard C, Caslake MJ, Soro-Paavonen A, Westerbacka J, et al. Overproduction of large VLDL particles is driven by increased liver fat content in man. Diabetologia. 2006. 49:755–765.

34. Boden G, Shulman GI. Free fatty acids in obesity and type 2 diabetes: defining their role in the development of insulin resistance and beta-cell dysfunction. Eur J Clin Invest. 2002. 32 Suppl 3:14–23.
35. McGarry JD. Banting lecture 2001: dysregulation of fatty acid metabolism in the etiology of type 2 diabetes. Diabetes. 2002. 51:7–18.
36. Johansen T, Richelsen B, Hansen HS, Din N, Malmlöf K. Growth hormone-mediated breakdown of body fat: effects of GH on lipases in adipose tissue and skeletal muscle of old rats fed different diets. Horm Metab Res. 2003. 35:243–250.

37. Yip RG, Goodman HM. Growth hormone and dexamethasone stimulate lipolysis and activate adenylyl cyclase in rat adipocytes by selectively shifting Gi alpha2 to lower density membrane fractions. Endocrinology. 1999. 140:1219–1227.

38. Louveau I, Gondret F. Regulation of development and metabolism of adipose tissue by growth hormone and the insulin-like growth factor system. Domest Anim Endocrinol. 2004. 27:241–255.

39. Richelsen B. Effect of growth hormone on adipose tissue and skeletal muscle lipoprotein lipase activity in humans. J Endocrinol Invest. 1999. 22:5 Suppl. 10–15.
40. Randle PJ, Garland PB, Hales CN, Newsholme EA. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963. 1:785–789.

41. Cartee GD. What insights into age-related changes in skeletal muscle are provided by animal models? J Gerontol A Biol Sci Med Sci. 1995. 50 Spec No:137–141.
42. Christopher M, Hew FL, Oakley M, Rantzau C, Alford F. Defects of insulin action and skeletal muscle glucose metabolism in growth hormone-deficient adults persist after 24 months of recombinant human growth hormone therapy. J Clin Endocrinol Metab. 1998. 83:1668–1681.

43. Jessen N, Djurhuus CB, Jørgensen JO, Jensen LS, Møller N, Lund S, et al. Evidence against a role for insulin-signaling proteins PI 3-kinase and Akt in insulin resistance in human skeletal muscle induced by short-term GH infusion. Am J Physiol Endocrinol Metab. 2005. 288:E194–E199.

44. Boden G. Effects of free fatty acids (FFA) on glucose metabolism: significance for insulin resistance and type 2 diabetes. Exp Clin Endocrinol Diabetes. 2003. 111:121–124.

45. Goodpaster BH, Thaete FL, Kelley DE. Thigh adipose tissue distribution is associated with insulin resistance in obesity and in type 2 diabetes mellitus. Am J Clin Nutr. 2000. 71:885–892.

46. Stannard SR, Johnson NA. Insulin resistance and elevated triglyceride in muscle: more important for survival than "thrifty" genes? J Physiol. 2004. 554(Pt 3):595–607.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download