Abstract
Purpose
To determine whether insulin-like growth factor (IGF-1) affects transforming growth factor (TGF-β)-mediated fibronectin accumulation in human lens epithelial cell line (HLE B-3) cells.
Materials and Methods
HLE
B-3 cells were incubated for 24 hours with TGF-β (10 ng/mL), IGF-1 (10 ng/mL), or both. Expression of the fibronectin gene was determined using a real-time reverse transcriptase-polymerase chain reaction (RT-PCR). Fibronectin levels were examined using western blot analysis and immunofluorescence staining.
Results
Expression of the fibronectin gene was not different between the TGF-β/IGF-1 treated group and the TGF-β treated group (p = 0.116). However, western blot analysis demonstrated decreased fibronectin levels in human lens epithelial cells treated with TGF-β and IGF-1 compared to those treated with TGF-β only (p < 0.01). Immunofluorescence staining disclosed inhibition of TGF-β-induced fibronectin in the presence of IGF-1.
Transforming growth factor (TGF-β) has been shown to induce epithelial-mesenchymal transformation for in vitro subcapsular cataracts. It induces both morphological changes (spindle cell formation, capsular wrinkling, extracellular matrix accumulation) as well as the molecular markers (type I and III collagen, laminin, alpha-smooth muscle actin, fibronectin, and tenascin) that are characteristic of subcapsular cataracts.1-4 TGF-β is also now being examined as a causative factor in posterior capsule opacification, another growth condition of the lens which involves transdifferentiation of lens epithelial cells remaining after cataract surgery.5
Insulin-like growth factor (IGF-1) is implicated in mechanisms involving lens polarization, proliferation, and differentiation.6,7 However, no studies have demonstrated the effects of IGF-1 on fibronectin accumulation in human lens epithelial cells. The present study was undertaken to investigate the role of IGF-1 in the accumulation of TGF-β-mediated fibronectin in human lens epithelial cells.
Human lens epithelial cells (HLE B-3) were provided by Usha Andley, Ph.D., and maintained as previously described.8 The HLE B-3 cell cultures were plated in a 60 mm culture dish, allowed to reach 75 - 80% confluence, and the serum was then starved for 24 hours. Cell cultures were treated with 10 ng/mL of TGF-β1, 10 ng/mL of IGF-1 (R&D Systems, Minneapolis, MN, USA), or both in a serum free media. The treated cells were compared with control cultures that were incubated under identical conditions, but in the absence of TGF-β1 or IGF-1. After a 24 hour treatment, total RNA was isolated from the HCE B-3 cells using TRIzol as the extraction reagent (Gibco-Invitrogen, Carlsbad, CA, USA).9 Cells were used at passage five for all experiments.
cDNA synthesis (SuperScript III Reverse Transcriptase; Gibco-Invitrogen) required the use of 1 µg total RNA.10 Reverse-transcription products were then ready for use in real-time polymerase chain reaction (PCR) preparations. From the 20 µL total reverse transcription volume, 1 µL was used for each PCR amplification.
Real-time PCR amplification was performed in the presence of double-labeled fluorogenic probes (TaqMan probes; PE-Applied Biosystems, Foster City, CA, USA) that allow real-time relative quantification of gene expression. All probes used in this study were purchased from PE-Applied Biosystems. Amplification was performed in triplicate with 1 µL of cDNA in a total volume of 50 µL (TaqMan chemistry; Applied Biosystems). Assays were performed using an ABI Prism 7700 Sequence Detection System (Applied Biosystems). To evaluate relative quantification, the efficiency of the target gene amplification was compared with the efficiency of the GAPDH amplification.11 A non-template control was included in all experiments performed with real-time PCR to evaluate DNA contamination of the reagents used for amplification. None of the experiments resulted in a positive signal from the non-template control. The data were represented as the mean ± SD for the three independent experiments.
HLE B-3 cells were lysed in buffer (25 mM HEPES pH 7.5, 0.3 M NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 0.05% Triton X-100, 0.5 mM DL-Dithiothreitol, 0.4 mM PMSF, 2 µg/mL leupeptin, and 1 µg/mL pepstatin A). After centrifugation for 10 min at 1200 rpm, 20 µg of cellular protein was loaded onto 10% SDS-polyacrylamide gels. The protein bands were then transferred electrophoretically to Immobilion™-P (Millipore corp., Bedford, MA, USA) PVDF membranes. The membranes were incubated with blocking solution (TBS-Tween/5% skim dry milk) and incubated with rabbit anti-fibronectin antibody (1 : 1000 dilution; Sigma Chemical Co., St Louis, MO, USA) overnight at 4℃. After removing the antibodies and washing the reactive proteins, the membrane was treated with peroxidase-conjugated anti-rabbit IgG (1 : 5000 dilution; Amersham Pharmacia Biotech, Buckinghamshire, England). Detection was carried out using enhanced chemiluminescence (ECL) detection reagents (Amersham Pharmacia Biotech) on Hyperfilm ECL (Amersham Pharmacia Biotech). β-actin was used as an endogenous reference to determine the integrity of the protein in each sample. Each band was quantified by densitometric scanning of the gels. Data were presented as the mean ± SD of the three independent experiments.
HLE B-3 cells were fixed for 5 min in 3.7% formaldehyde and then permeabilized in PBS containing 0.5% Triton-X100 for 5 min. Following two 5 min rinses with PBS (pH 7.4), cells were incubated with rabbit anti-fibronectin antibody (1 : 100 dilution; Sigma Chemical Co.) overnight at 4℃. Cells were washed twice with PBS for 5 min each and then followed by incubation in TRITC-conjugated donkey anti-rabbit IgG (1 : 1000 dilution; Sigma Chemical Co.) for 1 hour at room temperature. Cells were washed with PBS, applied to the slides, and photographed under a Zeiss Axiophot fluorescence microscope. Results are representative of at least three independent experiments.
Expression of the fibronectin gene transcripts in HLE B-3 cells for four groups (Table 1) was determined by real-time PCR (Fig. 1). The use of real-time PCR demonstrated that the level of fibronectin gene expression significantly increased following TGF-β1 treatment (p < 0.01). However, no change was detected in the expression of the fibronectin mRNA with the IGF-1 treatment in HLE B-3 cells. The amount of fibronectin transcripts was not significantly different between the control group and the IGF-1 treatment group (p = 0.305). The level of fibronectin gene expression remained essentially unaltered following 24 hours of treatment with TGF-β1 and IGF-1 when compared to treatment with TGF-β1 only (p = 0.116). These results indicate that IGF-1 did not affect fibronectin mRNA expression in human lens epithelial cells.
Western blot analysis was performed on total proteins obtained from HLE B-3 cells to determine the effects of TGF-β1, IGF-1, or both on fibronectin protein levels. Equivalent β-actin (an internal housekeeping control for western blot analysis) bands were obtained. As shown in Fig. 2, fibronectin levels in HLE B-3 cells increased after 24 hours of TGF-β1 treatment (p < 0.01) when compared to untreated control cells. The amount of fibronectin was not significantly different between control and IGF-1 treatment groups (p = 0.135). However, after treatment with TGF-β1 and IGF-1, fibronectin decreased when compared to cells treated with TGF-β1 only. Quantification of each band through densitometric scanning showed a significant decrease in fibronectin for lens epithelial cells treated with TGF-β1 and IGF-1 when compared to cells treated with TGF-β1 only (p < 0.01) (Fig. 2). These results indicate that IGF-1 did not just affect fibronectin protein levels, but also decreased TGF-β1-mediated fibronectin accumulation in human lens epithelial cells.
Immunofluorescence staining of HLE B-3 cells using anti-fibronectin antibodies indicated that groups treated with TGF-β1 (Fig. 3B) demonstrated more fluorescence when compared to the untreated control cells (Fig. 3A). However, following treatment with TGF-β1 and IGF-1 (Fig, 3D), less fluorescence was detected when compared to cells treated with TGF-β1 only (Fig, 3B). These immunofluorescence findings are consistent with the results of the western blot analysis.
The pathogenesis of subcapsular cataracts, which includes anterior polar cataracts and after-cataracts, is believed to occur through the aberrant transdifferentiation of lens epithelial cells (LECs) into myofibroblast-like cells that may be responsible for the abnormal accumulation of extracellular matrix.4,12-14 Inappropriate TGF-β signaling in the anterior lens epithelial cells results in an epithelial-mesenchymal transition (EMT) that bears morphological and molecular resemblance to human cataracts, including anterior subcapsular (ASC) and posterior capsule opacification (PCO; also known as secondary cataract or after-cataract), which occurs following cataract surgery.15 According to studies using transgenic mice, cataracts induced by the expression of TGF-β1 have many similarities to human capsulolenticular cataracts, which include anterior subcapsular cataracts and anterior polar cataracts.16 These lenticular plaques are derived from the lens epithelium, and are comprised of spindle-shaped cells interspersed with accumulations of extracellular matrix. The results from our study demonstrate that TGF-β treatment of human lens epithelial cells (HLE-B3) induces fibronectin accumulation. Previous studies have demonstrated that fibronectin has been detected among subcapsular plaques in rat lens cultured with TGF-β as well as in LECs transformed into mesenchymal-like cells in type I collagen gel.4,17 Lee and Joo have shown, using western blot analysis, that TGF-β significantly increases fibronectin protein levels in bovine LECs.3 Our data also demonstrated that TGF-β significantly increases fibronectin protein levels for in vitro human lens epithelial cells.
Our results demonstrate the role of IGF-1 in TGF-β-mediated fibronectin accumulation in human lens epithelial cells, although IGF-1 has been reported to be involved in lens cell proliferation and differentiation.6,7 The effects of IGF-1 on fibronectin accumulation have not previously been recognized in lens epithelial cells. Fibronectin expression did not significantly increase due to IGF-1 at both mRNA and protein levels. However, western blot analysis showed reduced fibronectin protein levels with the TGF-β and IGF-1 treatment when compared to the TGF-β only treatment group. Immunofluorescence data using anti-fibronectin antibodies also showed decreased fluorescence with the TGF-β and IGF-1 treatment when compared to the TGF-β only treatment group. IGF-1 and TGF-β costimulation has been reported to markedly increase extracellular matrix proteins (collagen type I, fibronectin, and plasminogen activator inhibitor-1) in keloid fibroblasts when compared with the TGF-β only culture, although IGF-1 treatment alone had no stimulatory effect.18 These results indicate different effects of IGF-1 on TGF-β-mediated fibronectin accumulation based on the cell type and organ.
By using a real-time RT-PCR, we demonstrated that IGF-1 did not alter the expression of the fibronectin gene in human lens epithelial cells. Fibronectin gene expression levels were not significantly different between the TGF-β1/IGF-1 treatment group and the TGF-β1 only treatment group. However, following the TGF-β1 and IGF-1 treatment, fibronectin protein levels were significantly reduced when compared to the TGF-β1 only treatment. We speculate that cross-communication between intracellular signal transduction pathways of TGF-β1 and IGF-1 may cause a discrepancy between mRNA and protein levels. Intracellular signal molecules from IGF-1 may affect the translation level of increased fibronectin mRNA or increased fibronectin gene stability from TGF-β1. Further studies are needed to investigate the molecular signaling mechanisms between TGF-β1 and IGF-1.
In summary, we have reported that IGF-1 counteracts TGF-β-mediated fibronectin accumulation in human lens epithelial cells. IGF-1 may have therapeutic uses due to its inhibitory effect on fibronectin accumulation in human lens epithelial cells.
Figures and Tables
 | Fig. 1The real-time polymerase chain reaction (PCR) demonstrated that no change was detected in the expression of the fibronectin gene following 24 hour treatment with insulin-like growth factor (IGF)-1 in human lens epithelial cells (HLE B-3). The amount of fibronectin transcripts significantly increased following treatment with TGF-β1. There was no significant difference in the amount of fibronectin (FN) gene transcripts between control and IGF-1 treatment groups, and between TGF-β1 only and TGF-β1 and IGF-1 treatment groups. The graphs show the mean values of the three independent experiments with the error bars representing the standard deviation. |
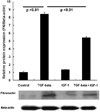 | Fig. 2IGF-1 counteracts TGF-β-mediated fibronectin accumulation in the human lens epithelial cell line (HLE B-3 cells). HLEB-3 cells in serum-free media were incubated for 24 hours with TGF-β1 (10 ng/mL), IGF-1 (10 ng/mL), or both. Fibronectin levels increased following treatment with TGF-β1 (10 ng/mL) in the western blot analysis. However, fibronectin (FN) levels decreased after treatment with TGF-β1 (10 ng/mL) and IGF-1 (10 ng/mL) when compared to treatment with TGF-β1 (10 ng/mL) only. Values in these graphs represent the mean of the three experiments and error bars represent the standard deviation. Values were normalized to the density of the respective β-actin band. |
 | Fig. 3Comparative fluorescent micrographs showing IGF-1 inhibition of TGF-β-mediated fibronectin accumulation in HLE B-3 cells. Cells were maintained (A) in serum free media alone; (B) treated with 10 ng/mL TGF-β1 for 24 hr; (C) treated with 10 ng/mL IGF-1 for 24 hr; (D) treated with 10 ng/mL IGF-1 in the presence of 10 ng/mL TGF-β1 for 24 hr. The bar represents 50 µm in A-D. |
References
1. Hales AM, Schulz MW, Chamberlain CG, McAvoy JW. TGF-beta1 induces lens cells to accumulate alpha-smooth muscle actin, a marker for subcapsular cataracts. Curr Eye Res. 1994. 13:885–890.

2. Hales AM, Chamberlain CG, McAvoy JW. Cataract induction in lenses cultured with transforming growth factor-beta. Invest Ophthalmol Vis Sci. 1995. 36:1709–1713.
3. Lee EH, Joo CK. Role of transforming growth factor-beta in transdifferentiation and fibrosis of lens epithelial cells. Invest Ophthalmol Vis Sci. 1999. 40:2025–2032.
4. Lovicu FJ, Schulz MW, Hales AM, Vincent LN, Overbeek PA, Chamberlain CG, et al. TGF-beta induces morphological and molecular changes similar to human anterior subcapsular cataract. Br J Ophthalmol. 2002. 86:220–226.

5. Cobo LM, Ohsawa E, Chandler D, Arguello R, George G. Pathogenesis of capsular opacification after extracapsular cataract extraction: An animal model. Ophthalmology. 1984. 91:857–863.

6. Lang RA. Which factors stimulate lens fiber cell differentiation in vivo? Invest Ophthalmol Vis Sci. 1999. 40:3075–3078.
7. Klok E, Lubsen NH, Chamberlain CG, McAvoy JW. Induction and maintenance of differentiation of rat lens epithelium by FGF-2, insulin and IGF-1. Exp Eye Res. 1998. 67:425–431.

8. Andley UP, Rhim JS, Chylack LT Jr, Fleming TP. Propagation and immortalization of human lens epithelial cells in culture. Invest Ophthalmol Vis Sci. 1994. 35:3094–3102.
9. Shin SY, Lee HJ, Ko DS, Lee HC, Park WI. The regulators of VEGF expression in mouse ovaries. Yonsei Med J. 2005. 46:679–686.

10. Chung SH, Lee SK, Cristol SM, Lee ES, Lee DW, Seo KY, et al. Impact of short-term exposure of commercial eyedrops preserved with benzalkonium chloride on precorneal mucin. Mol Vis. 2006. 12:415–421.
11. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods. 2001. 25:402–408.

12. Novotny GEK, Pau H. Myofibroblast-like cells in human anterior capsular cataract. Virchows Arch. 1984. 404:393–401.

13. Hatae T, Ishibashi T, Yoshitomi F, Shibata Y. Immunocytochemistry of types I-IV collagen in human anterior subcapsular cataracts. Graefes Arch Clin Exp Ophthalmol. 1993. 231:586–590.

14. Font RL, Brownstein S. A light and electron microscopic study of anterior subcapsular cataracts. Am J Ophthalmol. 1974. 78:972–984.

15. de Iongh RU, Wederell E, Lovicu FJ, McAvoy JW. Transforming growth factor-beta-induced epithelial-mesenchymal transition in the lens: a model for cataract formation. Cells Tissues Organs. 2005. 179:43–55.

16. Srinivasan Y, Lovicu FJ, Overbeek PA. Lens-specific expression of transforming growth factor beta1 in transgenic mice causes anterior subcapsular cataracts. J Clin Invest. 1998. 101:625–634.

17. Zuk A, Hay ED. Expression of beta1 integrins changes during transformation of avian lens epithelium to mesenchyme in collagen gels. Dev Dyn. 1994. 201:378–393.

18. Daian T, Ohtsuru A, Rogounovitch T, Ishihara H, Hirano A, Akiyama-Uchida Y, et al. Insulin-like growth factor-I enhances transforming growth factor-beta-induced extracellular matrix protein production through the P38/activating transcription factor-2 signaling pathway in keloid fibroblasts. J Invest Dermatol. 2003. 120:956–962.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download