Abstract
Purpose
We sought to evaluate the clinical usefulness of CT colonography (CTC) after incomplete conventional colonoscopy (CC) for occlusive colorectal cancer (CRC) according to the tumor location.
Materials and Methods
Seventy-five patients with occlusive CRC underwent subsequent CTC immediately after incomplete CC. Fifty-nine patients had distal CRC and 16 had proximal colon cancer. Experienced radiologists prospectively analyzed the location, length, and TNM staging of the main tumor. The colorectal polyps in the remaining colorectum and additional extraluminal findings were also recorded. Sixty-seven patients underwent colorectal resection. We retrospectively analyzed the surgical outcome and correlated CTC and CC findings.
Results
The overall accuracies of tumor staging were: T staging, 86%; N staging (nodal positivity), 70% (80%); and intra-abdominal M staging, 94%. Additional colonic polyps were found in 23 patients. Six synchronous carcinomas were detected (9%); three in the proximal colon and three in the distal colon of occlusion. Clinically significant localization errors at CC were noted in 8 patients (12%, 5 proximal colon cancers and 3 distal CRCs) and were corrected by CTC. After CTC, the surgeons modified the initial surgical plan in 11 cases (16%).
Conventional colonoscopy (CC) is the current standard technique for evaluating the entire colon. Full colonic evaluation is especially important in patients with colorectal malignancy because of the high prevalence of synchronous adenomas and carcinomas. At the same time, CC fails to show the entire colon in about 6 - 26% of cases, mainly due to occlusive colorectal cancer (CRC).1-4 About 15% of patients with CRC present with large bowel obstruction. In this situation, double-contrast barium enema (DCBE) might be the next choice for complete evaluation of the proximal colon.5,6 This method may be limited, however, by poor coating of the distended proximal bowel, low accuracy in detecting small polyps, and a problem of residual barium during surgery. Currently, computed tomography colonography (CTC) is regarded as a promising technique for complete evaluation of the proximal colon and simultaneous assessment of extraluminal status.7-9
Several reports discuss the usefulness of CTC immediately after incomplete CC due to occlusive CRC, and most focus on cases of distal colon or rectal carcinoma. These promising results have promoted CTC as a choice for preoperative evaluation after incomplete CC due to distal occlusive CRC. However, to our knowledge, no report has documented the usefulness of CTC according to tumor location, particularly for proximal occlusive colon cancers. The aim of this study was to evaluate the clinical usefulness of CTC in cases of incomplete CC due to occlusive CRC and to compare the results of CTC in proximal versus distal occlusive CRCs.
From Mar 2002 to Feb 2004, 75 patients with occlusive CRC underwent subsequent CTC immediately after incomplete CC. Experienced internists with gastrointestinal subspecialty training performed CC. Bowel cleansing was provided by ingesting 4 L of a polyethylene glycol electrolyte solution in a standard manner before the procedure. Initially, the patients were divided into two groups according to the colonoscopic finding: proximal colon cancer (cancer from the cecum to splenic flexure) and distal colon and rectal cancer (descending-sigmoid colon cancer, rectosigmoid, and rectal cancer, but not anal cancer). The occlusion site, cause of occlusion, and size and number of other colorectal polyps distal to the occlusion were recorded.
Seven patients with peritoneal dissemination of the malignancy and one patient with extensive hepatic metastasis were excluded. Ultimately, 67 patients (14 proximal and 53 distal occlusive CRC) with 73 histopathologically proven carcinomas were included in this study, consisting of 45 men and 22 women (mean age of 58.2 years with a range of 19 - 86 years). Informed consent was obtained from all patients and all examinations were performed in accordance with the recommendations of our institutional review board. Follow-up CC was carried out in 37 patients with surgically treated occlusive CRC.
CTC was performed with a 4-channel multi-detector row CT scanner (GE medial; LightSpeed). After injection of buscopan (hyoscine n-buthylbromide), room air was carefully insufflated using a manual balloon pump through a rectal enema tube according to the patient's tolerance. Air filling and distension of the colon were evaluated on the CT scout before CTC. Once bowel distention was adequate, CTC was performed after power injection of 120 mL (3 mL/sec; scanning delay, 60 sec) of an iodinated IV contrast agent. Two sets of images, one obtained with the patient supine and the other with the patient prone, were generated. CT parameters included 4 × 2-mm detector collimation, 120 kV, 150 - 200 mAs, and a pitch of 1.25. Axial CT images were reconstructed as 3-mm slices with a 1.5-mm reconstruction interval. CT images were transferred to a remote PC-based workstation using commercially available software (Rapidia; Infinitt). The processed images included multiplanar reformatted, ray-sum, and virtual colonoscopy images.
The experienced gastrointestinal radiologist prospectively analyzed tumor localization (tumor location and the length of the involved segment), and TNM staging of the main tumor, using TNM classification in the 6th edition of the AJCC and UICC system for rating cancer of the colon and the rectum from 2002 (Sobin and Wittekind, 2002). Colorectal polyps in the remaining colorectum proximal and distal to the obstruction were assessed. Additional extraluminal findings were also recorded. Postoperative CC was performed to control the CTC findings in the proximal part of the colon (n = 35).
Surgical resection was performed after multi-disciplinary team planning with surgeons, internists, and a radiologist. We retrospectively analyzed the surgical outcome and correlated CTC findings with the histopathologic findings and preoperative/postoperative CC results, including the accuracy of CTC for TNM staging (n = 73), accuracies of CTC and CC in localization of the main tumor (n = 67), and accuracies of CTC and CC in polyp detection, distal (n = 75) versus proximal (n = 35) to the obstruction. In polyp detection by CTC, sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of CTC were calculated, per person and per lesion.
Complete CTC examination was achieved in all 75 patients who underwent CTC immediately after incomplete CC due to occlusive colon cancer. Sixteen patients had proximal colon cancer and 59 patients had distal CRC. Seventy-three adenocarcinomas were retrieved from 67 patients. On histopathological examination, 12 of 73 tumors (16%) were staged as pT1/2, 53 (73%) as pT3, and 8 (11%) as pT4; 28 of 73 (38%) were staged as pN0, 27 (37%) as pN1, and 18 (25%) as pN2; and 56 of 67 (84%) were staged as intra-abdominal pM0 and 11 (16%) as pM1.
The overall accuracies of TNM staging on CTC were T staging, 86%; N staging, 70%; and intra-abdominal M staging, 94% (Table 1). The overall accuracy for prediction of pericolic fat infiltration was 96%. In advanced CRC (≥ pT3), overstaging and understaging occurred in three and four patients, respectively. These cases were all incorrectly interpreted as to whether the tumor perforated the visceral peritoneum (T4) or not (T3). The overall accuracy for prediction of nodal positivity (involved or tumor-free) was 80%. Intra-abdominal metastatic lesions were found in 11 patients: 4 liver metastasis (n = 4), peritoneal dissemination of the malignancy (n = 6), and central nodal metastasis (n = 3) (2 had multifocal lesions). A small, subcapsular hepatic metastasis was found that was missed on CT. Small metastatic para-aortic nodes in another patient were also missed on CT preoperatively. In determination of peritoneal dissemination, one patient was over-staged and one was under-staged (Fig. 1).
In 14 patients (21%, 6 proximal colon cancers and 8 distal CRCs), the exact site of the cancer was initially misdiagnosed on CC. Localization errors were clinically insignificant in six of the patients, and the planned operations were carried out without modification. Clinically significant localization errors were noted in eight patients (12%, 5 proximal colon cancers and 3 distal CRCs), and confirmed at surgery. The surgical approach and extent in those patients were modified after CTC (Table 2).
A total of six synchronous carcinomas (9%) were confirmed: three in the colon proximal to the occlusion and three distal to the occlusion. All of them were correctly diagnosed preoperatively by CTC. Sixteen lesions (including 3 synchronous colorectal cancers) were detected distal to the occlusive cancer in 12 patients. The accuracy of CTC per patient in the distal group was 96%; sensitivity, 83%; specificity, 98%; PPV, 92%; and NNV, 97%. Forty-two abnormalities (including 3 synchronous colon cancers) were detected proximal to the occlusive cancer in 13 patients. The accuracy of CTC per patient in the proximal group was 92%; sensitivity, 87%; specificity, 95%; PPV, 93%; and NNV, 91%. Per lesion, the sensitivity of CTC was 100% for polyps at least 10 mm in diameter and 88% for polyps at least 6 mm in diameter (Fig. 2).
After CTC, the surgeons changed the initial surgical plan in 11 cases (16%), 3 proximal synchronous carcinomas and 8 clinically significant localization errors by CC (Fig. 3).
At present, CT is regarded as a routine procedure for preoperative evaluation in patients suspected of having advanced CRC.10-12 Mauchley et al.10 suggested that routine preoperative CT provides information that definitely alters treatment in 16% patients and is cost-effective. However, previous studies have shown disappointing accuracies in nodal staging, with a range of 22 - 73%. The accuracy of T staging by CT is also not satisfactory, ranging from 53 to 77%.12-16 Recent multi-detector row CT (MDCT) scanners allow thinner collimation, resulting in marked improvement of scanning resolution. Accordingly, MDCT with virtual endoscopy and/or multiplanar reformation could improve the accuracy of preoperative staging, up to 83 - 95% in T staging and 80 - 85% in N staging.17-19 In our study, the accuracy of T staging was 86%, which is comparable but not superior to previous MDCT results. According to the 6th international TNM classification, a tumor perforating the visceral peritoneum is newly classified into T4. To our knowledge, there have been no reports about CT accuracy in staging using this new classification. The visceral peritoneum is a thin serous membrane that is not clearly defined in normal CT conditions, which probably lowered the CT accuracy in our study.
Total large bowel evaluation is important in planning the treatment of patients with CRC because synchronous adenomas and adenocarcinomas are found in 14 - 48% and 2 - 9% of such cases, respectively.20-28 Although colonoscopy is regarded as the gold standard for the evaluation of the colon for colorectal tumors, it may be incomplete due to tumor obstruction, which is a frequent event in distal cancers.4,29 There have been some efforts to evaluate the whole colon proximally to an occlusion using imaging modalities, including CTC7-9 and MR colonography (MRC).30-32 The results of both CTC and MRC in occlusive CRC are encouraging and evaluation of the whole colon by CTC or MRC is reportedly effective. Most reports focus on 'distal' occlusive CRC7,9,32 and indicate that CTC (and/or MRC) is useful in that setting.
The utility of CTC in 'proximal' occlusive colon cancer remains controversial, because the majority of advanced right colon cancer cases require classical or extended right hemicolectomy. We believe that CTC can play an important role even in proximal occlusive colon cancer since proximal occlusive colon cancer detected by CC may not be actual proximal colonic lesions. We observed many cases in which lesions thought to be in the proximal colon were actually distal colonic lesions. Among eight clinically significant localization errors, five were located in the proximal colon. There are some reports that CC has a considerable error rate for localization of CRC and is inaccurate in 11 - 21% of cases.33-36 Anatomic variation and the absence of fixed internal landmarks make it difficult to localize the tumor accurately. Furthermore, in occlusive colon cancer, tumor localization may be more difficult, even for experienced endoscopists, because inferring the tumor location from the ileo-cecal valve is impossible. We found that CC was inaccurate for tumor localization in 21% of occlusive CRC cases, and there were clinically significant localization errors in 11% of occlusive CRC cases that required modification of surgical approach.
Accurate tumor localization for rectal carcinomas also has substantial clinical importance for preventing the inappropriate use of adjuvant therapy and determining the proper surgery, such as segmental sigmoid resection, low anterior resection, or abdominoperineal resection.36 Preservation of the anal sphincter is dependent on the distance between the lower edge of the tumor and the external sphincter and levator ani muscle. According to a surgery textbook,37 the rigid proctosigmoidoscope is recommended for measuring the distance of the lower edge of the tumor, because the flexible sigmoidoscope is not as accurate for this determination. In our opinion, CTC may provide an objective measurement of the distance of the tumor from the anal verge, which is mandatory for rectal surgery. Further study is necessary to prove this hypothesis.
CTC can provide knowledge of whole colorectal lesions, accurate tumor localization and tumor extent, tumor/nodal staging, and extra-colic abnormalities, which are critical for the proper management of patients with CRC. As a result, CTC may become a modality of choice for preoperative evaluation of all colorectal cancers. In conclusion, CTC is useful not only for evaluation of the proximal bowel in occlusive CRC, but also for accurate staging and tumor localization, which is informative for the surgeon. CTC is recommended when endoscopists encounter occlusive CRC, regardless of tumor location.
Figures and Tables
Fig. 1
A 39-year-old woman with mid transverse colon cancer. Axial CT scan reveals irregular wall thickening with luminal narrowing of the transverse colon with pericolic fat infiltration (arrow). The visceral peritoneum was not identifiable and no solid organ invasion was visible, which suggested stage T3. However, the pathologic stage of this lesion proved to be pT4 because of tumor invasion into the visceral peritoneum.
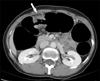
Fig. 2
A 44-year-old woman with proven cecal cancer. This occlusive lesion was initially thought to be in the transverse colon by the endoscopist. Three-dimensional surface rendering of the colon (A) and 2-dimensional coronal reformation (B) revealed a large fungating mass (arrows) in the cecum.
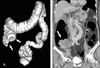
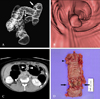
Fig. 3
A 45-year-old man with occlusive colon cancer in the splenic flexure (arrows) with a synchronous colon cancer in the mid transverse colon (arrowheads). (A) The virtual double-contrast display demonstrates an annular circumferential mass in the splenic flexure and a synchronous polypoid cancer in the mid transverse colon, proximal to the occlusion. (B) The transverse CT image and (C) endoluminal CT colonographic image clearly shows this synchronous malignant polyp. (D) Surgical extent was modified to an extended left hemicolectomy to include this synchronous lesion, which could not be identified by CC.
References
1. Nelson DB, McQuaid KR, Bond JH, Lieberman DA, Weiss DG, Johnston TK. Procedural success and complications of large-scale screening colonoscopy. Gastrointest Endosc. 2002. 55:307–314.

2. Cirocco WC, Rusin LC. Factors that predict incomplete colonoscopy. Dis Colon Rectum. 1995. 38:964–968.

3. Rex DK, Mark D, Clarke B, Lappas JC, Lehman GA. Colonoscopy evaluations: justification by cost? Am J Gastroenterol. 1996. 91:614–615.
4. Marshall JB, Barthel JS. The frequency of total colonoscopy and terminal ileal intubation in the 1990s. Gastrointest Endosc. 1993. 39:518–520.

5. Chong A, Shah JN, Levine MS, Rubesin SE, Laufer I, Ginsberg GG, et al. Diagnostic yield of barium enema examination after incomplete colonoscopy. Radiology. 2002. 223:620–624.

6. Brown AL, Skehan SJ, Greaney T, Rawlinson J, Somers S, Stevenson GW. Value of double-contrast barium enema performed immediately after incomplete colonoscopy. AJR Am J Roentgenol. 2001. 176:943–945.

7. Neri E, Giusti P, Battolla L, Vagli P, Boraschi P, Lencioni R, et al. Colorectal cancer: role of CT colonography in preoperative evaluation after incomplete colonoscopy. Radiology. 2002. 223:615–619.

8. Morrin MM, Farrell RJ, Raptopoulos V, McGee JB, Bleday R, Kruskal JB. Role of virtual computed tomographic colonography in patients with colorectal cancers and obstructing colorectal lesions. Dis Colon Rectum. 2000. 43:303–311.

9. Fenlon HM, McAneny DB, Nunes DP, Clarke PD, Ferrucci JT. Occlusive colon carcinoma: virtual colonoscopy in the preoperative evaluation of the proximal colon. Radiology. 1999. 210:423–428.

10. Mauchley DC, Lynge DC, Langdale LA, Stelzner MG, Mock CN, Billingsley KG. Clinical utility and cost-effectiveness of routine preoperative computed tomography scanning in patients with colon cancer. Am J Surg. 2005. 189:512–517.

11. McAndrew MR, Saba AK. Efficacy of routine preoperative computed tomography scans in colon cancer. Am Surg. 1999. 65:205–208.
12. Scharling ES, Wolfman NT, Bechtold RE. Computed tomography evaluation of colorectal carcinoma. Semin Roentgenol. 1996. 31:142–153.

13. Balthazar EJ, Megibow AJ, Hulnick D, Naidich DP. Carcinoma of the colon: detection and preoperative staging by CT. AJR Am J Roentgenol. 1988. 150:301–306.

14. Thoeni RF. Colorectal cancer: cross-sectional imaging for staging of primary tumor and detection of local recurrence. AJR Am J Roentgenol. 1991. 156:909–915.

15. Gazelle GS, Gaa J, Saini S, Shellito P. Staging of colon carcinoma using water enema CT. J Comput Assist Tomogr. 1995. 19:87–91.

16. Zerhouni EA, Rutter C, Hamilton SR, Balfe DM, Megibow AJ, Francis IR, et al. CT and MR imaging in the staging of colorectal carcinoma: report of the Radiology Diagnostic Oncology Group II. Radiology. 1996. 200:443–451.

17. Filippone A, Ambrosini R, Fuschi M, Marinelli T, Genovesi D, Bonomo L. Preoperative T and N staging of colorectal cancer: accuracy of contrast-enhanced multi-detector row CT colonography-initial experience. Radiology. 2004. 231:83–90.

18. Chung DJ, Huh KC, Choi WJ, Kim JK. CT colonography using 16-MDCT in the evaluation of colorectal cancer. AJR Am J Roentgenol. 2005. 184:98–103.

19. Jin KN, Lee JM, Kim SH, Shin KS, Lee JY, Han JK, et al. The diagnostic value of multiplanar reconstruction on MDCT colonography for the preoperative staging of colorectal cancer. Eur Radiol. 2006. 16:2284–2291.

20. Arenas RB, Fichera A, Mhoon D, Michelassi F. Incidence and therapeutic implications of synchronous colonic pathology in colorectal adenocarcinoma. Surgery. 1997. 122:706–710.

21. Langevin JM, Nivatvongs S. The true incidence of synchronous cancer of the large bowel. A prospective study. Am J Surg. 1984. 147:330–333.
22. Chu DZ, Giacco G, Martin RG, Guinee VF. The significance of synchronous carcinoma and polyps in the colon and rectum. Cancer. 1986. 57:445–450.

23. Isler JT, Brown PC, Lewis FG, Billingham RP. The role of preoperative colonoscopy in colorectal cancer. Dis Colon Rectum. 1987. 30:435–439.

24. Evers BM, Mullins RJ, Matthews TH, Broghamer WL, Polk HC Jr. Multiple adenocarcinomas of the colon and rectum. An analysis of incidences and current trends. Dis Colon Rectum. 1988. 31:518–522.
25. Barillari P, Ramacciato G, De Angelis R, Gozzo P, Indinnimeo M, Valabrega S, et al. Effect of preoperative colonoscopy on the incidence of synchronous and metachronous neoplasms. Acta Chir Scand. 1990. 156:163–166.
26. Pagana TJ, Ledesma EJ, Mittelman A, Nava HR. The use of colonoscopy in the study of synchronous colorectal neoplasms. Cancer. 1984. 53:356–359.

27. Chen HS, Sheen-Chen SM. Synchronous and "early" metachronous colorectal adenocarcinoma: analysis of prognosis and current trends. Dis Colon Rectum. 2000. 43:1093–1099.
28. Takeuchi H, Toda T, Nagasaki S, Kawano T, Minamisono Y, Maehara Y, et al. Synchronous multiple colorectal adenocarcinomas. J Surg Oncol. 1997. 64:304–307.

29. Bat L, Neumann G, Shemesh E. The association of synchronous neoplasms with occluding colorectal cancer. Dis Colon Rectum. 1985. 28:149–151.

30. Ajaj W, Lauenstein TC, Pelster G, Holtmann G, Ruehm SG, Debatin JF, et al. MR colonography in patients with incomplete conventional colonoscopy. Radiology. 2005. 234:452–459.

31. Hartmann D, Bassler B, Schilling D, Pfeiffer B, Jakobs R, Eickhoff A, et al. Incomplete conventional colonoscopy: magnetic resonance colonography in the evaluation of the proximal colon. Endoscopy. 2005. 37:816–820.

32. Wong TY, Lam WW, So NM, Lee JF, Leung KL. Air-inflated magnetic resonance colonography in patients with incomplete conventional colonoscopy: Comparison with intraoperative findings, pathology specimens, and follow-up conventional colonoscopy. Am J Gastroenterol. 2007. 102:56–63.

33. Vignati P, Welch JP, Cohen JL. Endoscopic localization of colon cancers. Surg Endosc. 1994. 8:1085–1087.

34. Hancock JH, Talbot RW. Accuracy of colonoscopy in localisation of colorectal cancer. Int J Colorectal Dis. 1995. 10:140–141.

35. Lam DT, Kwong KH, Lam CW, Leong HT, Kwok SP. How useful is colonoscopy in locating colorectal lesions? Surg Endos. 1998. 12:839–841.

36. Piscatelli N, Hyman N, Osler T. Localizing colorectal cancer by colonoscopy. Arch Surg. 2005. 140:932–935.

37. Corman ML. Carcinoma of the Rectum. Colon & Rectal Surgery. 2005. 5th ed. New York: Lippincott Williams & Wilkins;911–921.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download