Abstract
Purpose
Lamivudine is known to be very effective in suppressing hepatitis B virus replication and virus induced necroinflammation. The aim of this study was to evaluate lamivudine therapy efficacy, predictive factors, breakthrough, prevalence of YMDD mutation, and relapse rate in Korean children with chronic hepatitis B.
Materials and Methods
Between August 1999 and February 2005, 60 children on lamivudine therapy for chronic hepatitis B were enrolled. Treatment response was defined as alanine aminotransferase (ALT) normalization, and HBeAg and HBV-DNA disappearance.
Results
Seroconversion rates of HBeAg and HBV-DNA were 42% and 53%, respectively, and ALT normalization rate was 88%. Seroconversion rates of HBeAg (60.0%) and anti-HBe (60.0%) were higher in patients younger than 6 years. Seroconversion rate of HBV-DNA (68.4%) and normalization rate of serum ALT (94.7%) were highest in patients between 6 and 12 years. Seroconversion rates of all HBV markers were lowest in patients older than 12 years. Predicted 3 year cumulative seroconversion rates, were 70%, 68% for HBeAg, HBV-DNA, respectively. These were calculated by Kaplan-Meier method. Cox proportional hazard regression model showed that pre-treatment ALT was a positive predictive factor for seroconversion of HBeAg and HBV-DNA. Breakthrough phenomenon was noted in 6 patients, and 3 had a YMDD mutation.
Worldwide, 2 billion people have been infected with hepatitis B virus (HBV) and more than 350 million are chronic carriers. Approximately 25% to 40% of carriers will develop cirrhosis or hepatocellular carcinoma (HCC).1 In Korea, Choi et al.2 reported a 12.3% carrier rate in 1983. With introduction of HBV vaccination, the carrier rate decreased to 0.4 - 0.7%.3,4 Treatment with early anti-viral therapy aims to reduce complications, including liver cirrhosis and hepatocellular carcinoma.
Previous anti-viral and immunosuppressive treatments for HBV were ineffective and discontinued. Current anti-viral treatment includes interferon-alpha (IFN) and lamivudine. Lamivudine is the derivative of pyrimidine, and although originally developed as an anti-viral for human immunodeficiency virus (HIV), was found to selectively repress HBV-DNA polymerase.5 A disadvantage of lamivudine is that HBV-DNA mutation can develop, and emergence of the wild type virus can occur after drug cessation.6-8 The present study aims to assess the efficacy of lamivudine therapy, breakthrough, prevalence of YMDD mutation, and the relapse rate in children with chronic hepatitis B.
Sixty patients diagnosed with chronic hepatitis B at Severance Children Hospital, Yonsei University College of Medicine, were enrolled in this cohort study between August 1999 and February 2005. Inclusion criteria for lamivudine therapy included increased serum aminotransferase activity (at least two times the upper normal limit) and a minimum 6 month presence of HBsAg, HBeAg, and HBV-DNA markers. Liver biopsies were not performed. All patients were treated with lamivudine (Glaxo Wellcome Research and Development Ltd., Greenford, England). Serum total bilirubin (T.bil), alkaline phosphatase (ALP), cholesterol, albumin, AST, ALT, HBeAg, anti-HBe, and HBV-DNA were measured every 3 to 6 months. Enzyme immunoassay (Abbott Laboratories, North Chicago, IL) was used to examine HBeAg and anti-HBe. Solution hybridization technique (Hybrid Capture; Digene Diagnostics, Beltsville, MD, USA) was used to detect HBV-DNA, and values greater than 0.5 pg/dL (> 141,500 copies/mL using RT-PCR) were considered positive. Positive response to lamivudine therapy was defined as seroconversion of HBeAg and anti-HBe, undetectable serum HBV-DNA, and normalization of serum aminotransferase. Patients were divided into 3 groups based on virologic and serologic response to therapy, which were responders (normalized HBeAg, anti-HBe, HBV-DNA, and serum aminotransferase), partial responders (normalized serum aminotransferase but only HBeAg or HBV-DNA seroconversion), and non-responders (no change in HBeAg, anti-HBe, and HBV-DNA). In patients with breakthrough (ALT increased more than two times the upper normal limit and HBV-DNA greater than 141,500 copies/mL using PCR), YMDD mutation was evaluated by using nested polymerase chain reaction and restriction-fragment-length polymorphism assay.9 Many reports in Korea said that genotype C HBV prevails predominantly among chronic carriers of the virus in Korea (80 - 100%), irrespective of their age, clinical stages of liver disease and geographic origin.10-13 So HBV genotyping was skipped because of previous reports.
Patients who visited hospital had each deferent initiation time of lamivudine. Because of out national insurance, we could not define the duration of therapy. SPSS (version 12.0) was used for statistical analysis. To evaluate long-term treatment results, Kaplan-Meier method was used to predict the cumulative seroconversion rate. Cox proportional hazard regression model was used to evaluate correlations between HBV marker seroconversion and age, gender, serum ALT, and HBV-DNA. Frequency and cross-tabulation analysis were also used. Results were considered significant at the p < 0.05 level.
Sixty patients were diagnosed with chronic hepatitis B between August 1999 and February 2005. The mean duration of therapy was 16.4 ± 7.3 months, and the mean follow-up duration was 23.8 ± 28.0 months. Twenty-four (40%) patients were considered responders, 8 (13.3%) partial responders, and 28 (47.7%) non-responders. The pre-treatment ALT and AST values of the responder group were significantly higher compared to other groups (p = 0.013, p = 0.020, respectively). There was no significant difference of serum HBV-DNA among the 3 groups (p = 0.08) (Table 1). Family history of chronic hepatitis B was noted in 81.7% of children.
Following lamivudine therapy, seroconversion of HBV-DNA, HBeAg, and anti-HBe were noted in 32 (53%), 25 (42%), and 25 (42%) patients, respectively. Seroconversion of both HBeAg and HBV-DNA, HBV-DNA only, and HBeAg only occurred in 24 (40%), 8 (13%), and 1 (1.7%) patient, respectively. Serum ALT normalized in 53 (88%) patients (Table 2). The mean time to seroconversion for HBV-DNA, HBeAg, and anti-HBe was 7.2 ± 6.4, 11.2 ± 8.1, and 11.4 ± 7.9 months, respectively. Normalization of serum ALT took 5.3 ± 5.2 months (Table 3). Seroconversion rates of HBeAg (60.0%) and anti-HBe (60.0%) were higher in patients younger than 6 years. Seroconversion rate of HBV-DNA (68.4%) and normalization rate of serum ALT (94.7%) were highest in patients between 6 and 12 years. Seroconversion rates of all HBV markers were lowest in patients older than 12 years (Table 4). Seroconversion rates of HBeAg (63.6%), anti-HBe (63.6%), and HBV-DNA (72.7%) were higher in patients with pre-treatment ALT values > 300 IU/dL (Table 5). Seroconversion rates of HBeAg (56.5%), anti-HBe (60.9%) and HBV-DNA (65.2%) were higher in patients with pretreatment HBV-DNA < 500 pg/dL (Table 6).
The predicted cumulative seroconversion rate for 3 years after initiation of lamivudine therapy was calculated using Kaplan-Meier method. Predicted cumulative seroconversion rates of HBeAg, anti-HBe, and HBV-DNA were 70%, 65%, and 68%, respectively (Fig. 1, 2, and 3). All cumulative seroconversion rates were sustained until 5 years after initiation of therapy.
The effects of pre-treatment age, gender, and serum ALT and HBV-DNA on seroconversion rates were assessed with Cox proportional hazard regression model. Age, gender, and pre-treatment HBV-DNA had no effect on seroconversion rate of HBeAg, and anti-HBe. Seroconversion rate of HBeAg, anti-HBe, and HBV-DNA were significantly higher in patients with elevated pre-treatment serum ALT (p = 0.018, 0.028, and 0.003) (Table 7).
During therapy, only 6 (10%) patients had breakthrough phenomenon of serum ALT and HBV-DNA. Among these patients, 3 had YMDD mutation, with breakthroughs occurring at 12, 13, and 22 months after initiation of lamivudine. Of the 3 (5%) patients, 2 had both YIDD (methionine → isoleucine) and L528M mutations, and 1 had only YIDD mutation. Relapse occurred in 2 (3.3%) patients. In 1 patient, seroconversion occurred at 14 months after initiation of therapy, with redetection of HBV-DNA at 21 months. In the other patient, seroconversion occurred at 6 months therapy with HBV-DNA detection at 16 months.
Lamivudine is effective for repression of necroinflammation, liver fibrosis, seroconversion of serum HBV-DNA and HBeAg, and normalization of serum ALT.6,14,15
Sokal et al.16 reported mean seroconversion rates of 48% and 35% for HBV-DNA and HBeAg in patients 2 to 17 years following 1 year of lamivudine. Jonas et al.17 reported 61% and 26% seroconversion rates of HBV-DNA and HBeAg in patients 2 to 17 years following treatment with 3 mg/kg for 52 weeks. Ozgenc et al.18 reported that seroconversion was achieved in 15.6% (7/45) and 5.6% (2/36) at the end of first and second year, and 0% (none) at the end of third and fourth year with lamivudine therapy, respectively. In our study, mean seroconversion rates of HBV-DNA and HBeAg were 53% and 42% in Korean patients 2 to 17 years, respectively, following treatment with 3 mg/kg for varying duration. Using Kaplan-Meier method, predicted cumulative seroconversion rates at 3 years were 68% and 70% for HBV-DNA and HBeAg, respectively.
Sokal et al.16 reported a 40% and 24% HBeAg seroconversion rate HBeAg in patients younger than 7 years and aged 7 to 12 years, respectively. Similarly, in this study, the seroconversion rate was 60% in Korean patients younger than 6 years, and 42.1% in Korean patients 6 to 12 years. Liberek et al.19 studied 59 patients with chronic hepatitis B aged 6 to 18 years. They reported a significant influence of pre-treatment serum HBV-DNA (p = 0.001) on seroconversion rates for HBV-DNA and HBeAg, however age (p = 0.07), gender (p = 0.72), and pre-treatment serum ALT (p = 0.14) had no influence. Alexander et al.20 studied 60 patients with chronic hepatitis B aged 4 to 80 years. They reported a significant influence of pre-treatment serum ALT (p = 0.002) and HBV-DNA (p = 0.004) on seroconversion rates for HBeAg, however age, gender, and liver cirrhosis had no influence. In this study, pre-treatment serum ALT had a significant influence on seroconversion of HBV-DNA (p = 0.03), HBeAg (p = 0.018) and anti-HBe (p = 0.028), but not on age, gender, or pre-treatment HBV-DNA.
In lamivudine therapy group previously treated with placebo, Sokal et al.16 reported YMDD mutation incidence increased from 0% at baseline, to 19% at 12 months, and to 49% at 24 months. In lamivudine therapy group previously treated with lamivudine, YMDD mutation incidence increased from 24% at baseline, to 59% at 12 months, and to 64% at 24 months. Ozgenc et al.18 reported that breakthrough incidence was detected in six (13.3%) cases at 12 months and increased to 69.4% (n = 25) and 82.4% (n = 14) at the end of the second and third years with lamivudine therapy, respectively. Hartman et al.21 reported that YMDD mutants were detected in 11 of 17 (65%) children after 1 year of lamivudine treatment. Among children with YMDD mutant variants, 54% maintained normal ALT values and 45% had undetectable HBV DNA by hybridization assay. In this study, breakthrough phenomenon developed in only 6 (10%) Korean patients; 3 (5%) had YMDD mutation at 12, 13, and 22 months following lamivudine initiation. YIDD mutation (methionin → isoleucine) and L528M mutation were present in 2 of the 3, and the other patient had only YIDD mutation. Patients with breakthrough received adefovir. We suspected the reason of lower incidence of YMDD mutation might be caused by somewhat geographical, epidemiological, and genetic differences, but exactly did not know the reason. So we had a plan to evaluate the genotype in the next report and liver biopsies were not performed. Relapse occurred in 2 (3.3%) Korean patients. One patient seroconverted at 14 months with HBV-DNA reoccurrence at 21 months following initiation. The other patient seroconverted at 6 months and had HBV-DNA reoccurrence at 16 months.
In conclusion, our study showed that pre-treatment ALT was an important factor influencing virologic response. The seroconversion rate for HBV-DNA, HBeAg, breakthrough, YMDD mutation, and relapse was 53%, 42%, 10%, 5%, and 3.3%, respectively. Despite high seroconversion rates in Korean children treated with lamivudine, new therapeutic agents are needed for improved viral suppression and reduction of emergence of resistance.
Figures and Tables
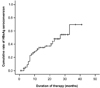 | Fig. 1Predictive cumulative rate of seroconversion of HBeAg analyzed by Kaplan-Meier method in patients with chronic hepatitis B. Approximately, a 70% seroconversion rate can be predicted at year 3. |
 | Fig. 2Predictive cumulative rate of seroconversion of HBeAg analyzed by Kaplan-Meier method in patients with chronic hepatitis B. Approximately, a 65% seroconversion rate can be predicted at year 3. |
 | Fig. 3Predictive cumulative rate of seroconversion of HBeAg analyzed by Kaplan-Meier method in patients with chronic hepatitis B. Approximately, a 68% seroconversion rate can be predicted at year 3. |
References
1. Yuen MF, Hui CK, Cheng CC, Wu CH, Lai YP, Lai CL. Long term follow-up of interferon alfa treatment in Chinese patients with chronic hepatitis B infection: The effect on hepatitis B e antigen seroconversion and the development of cirrhosis-related complications. Hepatology. 2001. 34:139–145.

2. Choi HJ, Kim YS, Park KS, Lee SI, Moon YM, Kang JK, et al. Clinical study on distribution of hepatitis B virus markers in Korean population. Korean J Gastroenterol. 1983. 15:163–171.
3. Jeong IS, Chung KS. The therapeutic effect of interferon-alpha treatment in children with chronic hepatitis B. Korean J Pediatr. 1997. 40:955–964.
4. Chang JY, Jeong SJ, Kim SK, Son BK, Hong YJ, Hong KS. Positive rate of HBsAg in school children in Incheon area. Korean J Pediatr Infect Dis. 2003. 10:153–158.

5. Mansour TS, Jin H, Wang W, Hooker EU, Ashman C, Cammack N, et al. Anti-human immunodeficiency virus and anti-hepatitis-B virus activities and toxicities of the enatiomers of 2'-deoxy-3'-oxa-4'-thiocytidine and their 5-fluoro analogues in vitro. J Med Chem. 1995. 38:1–4.

6. Lai CL, Chien RN, Leung NW, Chang TT, Guan R, Tai AI, et al. A one-year trial of lamivudine for chronic hepatitis B. Asia Hepatitis Lamivudine Study Group. N Engl J Med. 1998. 339:61–68.

7. Honkoop P, Niesters HG, de Man RA, Osterhaus AD, Schalm SW. Lamivudine resistance in immunocompetent chronic hepatitis B. Incidence and patterns. J Hepatol. 1997. 26:1393–1395.
8. Chayama K, Suzuki Y, Kobayashi M, Kobayashi M, Tsubota A, Hashimoto M, et al. Emergence and takeover of YMDD motif mutant hepatitis B virus during long term lamivudine therapy and re-takeover by wild type after cessation of therapy. Hepatology. 1998. 27:1711–1716.

9. Allen MI, Gauthier J, DesLauriers M, Bourne EJ, Carrick KM, Baldanti F, et al. Two sensitive PCR-based methods for detection of hepatitis B virus variants associated with reduced susceptibility to lamivudine. J Clin Microbiol. 1999. 37:3338–3347.

10. Cha CH, Sohn YH, Jang SS, Lee HJ, Lee KJ, Shin ES, et al. Genotype analysis of hepatitis B virus isolated from Korean hepatitis patients. Korean J Lab Med. 2003. 23:352–356.
11. Chung JY, Han TH, Hwang ES, Ko JS, Seo JK. Prevalence and genotype of transfusion-transmitted virus in children with hepatitis and normal control. Korean J Pediatr Gastroenterol Nutr. 2005. 8:202–212.

12. Bae SH, Yoon SK, Jang JW, Kim CW, Nam SW, Choi JY, et al. Hepatitis B virus genotype C prevails among chronic carriers of the virus in Korea. J Korean Med Sci. 2005. 20:816–820.

13. Kim JH, Koh DK, Hur JK, Kang JH, Nainan OV, Margolis HS. The incidence rate of hepatitis B virus surface gene variants in Korean children with immunoprophylaxis failure of perinatal infection. Korean J Hepatol. 2005. 11:320–328.
14. Dienstag JL, Schiff ER, Wright TL, Perrillo RP, Hann HW, Goodman Z, et al. Lamivudine as initial treatment for chronic hepatitis B in the United States. N Engl J Med. 1999. 341:1256–1263.

15. Schalm SW, Heathcote J, Cianciara J, Farrell G, Sherman M, Willems B, et al. Lamivudine and alpha interferon combination treatment of patients with chronic hepatitis B infection: a randomised trial. Gut. 2000. 46:562–568.

16. Sokal EM, Mizerski J, Badia IB, Areias JA, Schwarz KB, Little NR, et al. Long-term lamivudine therapy for children with HBeAg-positive chronic hepatitis B. Hepatology. 2006. 43:225–232.

17. Jonas MM, Mizerski J, Badia IB, Areias JA, Schwarz KB, Little NR, et al. Clinical trial of lamivudine in children with chronic hepatitis B. N Engl J Med. 2002. 346:1706–1713.

18. Ozgenç F, Arikan C, Sertoz RY, Nart D, Aydogdu S, Yagci RY. Effect of long-term lamivudine in chronic hepatitis B virus-infected children. Antivir Ther. 2004. 9:729–732.

19. Liberek A, Szaflarska-Poplawska A, Korzon M, Luczak G, Góra-Gebka M, Loś-Rycharska E, et al. Lamivudine therapy for children with chronic hepatitis B. World J Gastroenterol. 2006. 12:2412–2416.





 PDF
PDF ePub
ePub Citation
Citation Print
Print









 XML Download
XML Download