Abstract
Moyamoya disease is an occlusive intracranial arteriopathy owing to intimal hyperplasia with formation of abnormal cerebrovascular collateral networks; however, the etiology remains unclear. Although this disease is known to be associated with renovascular hypertension, it is extremely rare for it to be associated with stenoses of the coronary arteries. We herein described a case of a 56-year-old female with angina and asymptomatic moyamoya disease. We performed off-pump coronary artery bypass grafting (OPCAB) to avoid cardiopulmonary bypass and the risk of intraoperative hypotension. Conventional coronary artery bypass grafting has a potential risk of brain ischemia in moyamoya patients, but OPCAB may avoid this perioperative cerebral ischemic complication.
Moyamoya disease is a progressive cerebrovascular occlusive disease, occurring predominantly in the Northeastern Asian countries. Although the etiology remains unclear, it is characterized by intracranial stenosis or occlusion of the internal carotid arteries, with bilateral abnormal vascular networks at the base of the brain.1 Clinically, ischemic events occur mainly in childhood, while hemorrhagic events occur in adulthood.2 Thus, moyamoya disease has a bimodal incidence. In the literature, extracranial vascular involvement has been reported mainly in the renal artery.3 However, coronary artery involvement among patients with moyamoya disease is extremely rare. Such patients have a potential risk of perioperative brain ischemia if they require on-pump coronary artery bypass grafting (CABG). The recent advanced technique of off-pump coronary artery bypass grafting (OPCAB) can reduce the risk of complications by cardiopulmonary bypass (CPB). We successfully performed OPCAB on a patient who had coronary artery stenosis with moyamoya disease.
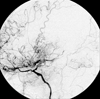
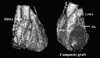
A 56-year-old female was referred to our hospital for recent effort aggravated angina. She had no history of hypertension. Approximately three years prior to admission, she was diagnosed with moyamoya disease at a check-up for general weakness at another institution. She had typical findings of angiographic features of definite type of Moyamoya disease (Fig. 1).4 One year after the diagnosis, she developed effort angina. Coronary angiography, performed at another hospital, revealed triple vessel disease. This coronary stenosis was treated by balloon angioplasty and stenting. However, these procedures failed to relieve symptoms, and the patient was referred to us for CABG. Neurologic examination showed no abnormal findings. Laboratory examination demonstrated no evidence of diabetes mellitus or hyperlipidemia. In consideration of the moyamoya disease, we used OPCAB in order to avoid the risk of brain ischemia associated with CPB. Intraoperative observation indicated that the stenoses were caused by atherosclerosis. The patient received a left internal mammary graft to the left anterior descending artery, a composite radial artery free graft to the first obtuse marginal artery, and a right internal mammary graft to the right posterolateral branch. During the operation, systolic blood pressure and blood PaCO2 level were constantly maintained above 130mmHg and 35 mmHg, respectively. The operation was uneventful and there was no perioperative cerebral ischemic episode; the patient had a stable course and was discharged nine days postoperatively.
Moyamoya disease is a rare entity of unclear cause that develops predominantly in Northeastern Asian people. Although this disease is usually confined to the intracranial cavity, it is sometimes associated with the extracranial arterial system, such as the renal or coronary artery. Although a combination of moyamoya and renovascular hypertension is well described, the association of coronary artery disease and moyamoya is rare. There is some evidence that it may be a systemic disease. Aoyage and colleagues showed that smooth muscle cells explanted from scalp arteries, which are remote from the arteries frequently involved, had altered cellular responses to platelet-derived growth factor in patients with moyamoya disease.6 Coronary artery stenosis in moyamoya disease may be caused either by fibrous intimal thickening, similar to the histopatholgical changes of the intracranial internal carotid arteries or atherosclerosis.7 In the previous literature, only twelve cases of moyamoya and associated coronary artery stenosis, have been reported.8 Among them, only three cases underwent coronary artery bypass grafting, and two of those had CPB. Only one case had CABG without CPB; a 56-year-old hypertensive Japanese female underwent minimally invasive direct CABG through a left anterior mini-thoracotomy for one vessel disease.7 However, our case had complex triple vessel disease, and required total arterial revascularization using OPCAB to avoid CPB and aortic manipulation. It is noteworthy to mention that our case was different from any other reported case.
CPB in moyamoya patients has a high risk of decreasing cerebral perfusion pressure due to the perfusion pressure variability in the initial stages of CPB and nonpulsatile flow. The risk of hypocapnic cerebral vasoconstriction and hypercapnic cerebral steal is well recognized.9 The most important point, for these patients who require coronary artery bypass grafting, is to avoid possible hypotension and maintain proper management of blood PaCO2 level. OPCAB can assist in these situations.
In conclusion, OPCAB is a safe procedure that avoids the risk of CPB related hypotensive brain ischemia, for multi-vessel coronary patients with moyamoya disease.
Figures and Tables
References
1. Yonekawa Y, Goto N. Barnett H, Mohr J, Stein B, Yatsu F, editors. Moyamoya disease: Diagnosis, treatment, and rescent achievement. Stroke: Pathophysiology, Diagnosis and management. 1992. 2nd ed. New York, NY: Churchill Livingstone;727–747.
3. Halley SE, White WB, Ramsby GR, Voytovich AE. Renovascular hypertension in moyamoya syndrome. Therapeutic response to percutaneous transluminal angioplasty. Am J Hypertens. 1988. 1:348–352.

4. Natori Y, Ikezaki K, Matsushima T, Fukui M. 'Angiographic moyamoya' its definition, classification, and therapy. Clin Neurol Neurosurg. 1997. 99:Suppl 2. S168–S172.

5. Moon JY, Chung N, Choi BW, Choe KO, Seo HS, Ko YG, et al. The utility of multi-detector row spiral CT for detection of coronary artery stenoses. Yonsei Med J. 2005. 46:86–94.

6. Aoyagi M, Fukai N, Sakamoto H, Shinkai T, Matsushima Y, Yamamoto M, et al. Altered cellular responses to serum mitogens, including platelet-derived growth factor, in cultured smooth muscle cells derived from arteries of patients with moyamoya disease. J Cell Physiol. 1991. 147:191–198.

7. Komiyama M, Ishikawa T, Takanashi S, Shimizu Y. Minimal invasive direct coronary artery bypass in moyamoya disease. Interact Cardiovasc Thorac Surg. 2003. 2:65–67.

8. Komiyama M, Nishikawa M, Yasui T, Otsuka M, Haze K. Moyamoya disease and coronary artery disease-case report. Neurol Med Chir (Tokyo). 2001. 41:37–41.




 ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download