Abstract
Purpose
Oxidative stress has been suggested to play a role as a common mediator of apoptosis and kidney damage in diabetes. However, it is uncertain whether the apoptosis occurs in the kidney during the course of diabetes. We investigated the occurrence of apoptosis in the diabetic rat kidney, the role of oxidative stress and the effect of an antioxidant on apoptosis in the diabetic rat kidney.
Materials and Methods
Otsuka-Long-Evans-Tokushima-Fatty rats, an animal model for type 2 diabetes, were randomized into a non-treated diabetic (n = 8) and a vitamin C-treated group (n = 8). Long-Evans Tokushima Otsuka rats (n = 8 ) were used as a control.
Results
Apoptosis was present in the epithelial cells of the proximal tubules in diabetic rats. The number of apoptotic cells, albuminuria, proteinuria, glomerular and tubulointerstitial sclerosis, and renal malondialdehyde were significantly decreased in vitamin C-treated diabetic rats when compared to the untreated diabetic rats. The decreased slit pore density (number of slit pores per underlying glomerular basement membrane length) as assessed by electron microscopy was also significantly restored by treatment with vitamin C without significantly affecting plasma glucose in diabetic rats.
Diabetic nephropathy is a leading cause of endstage renal disease. It is characterized functionally by proteinuria and albuminuria and pathologically by glomerular hypertrophy, mesangial expansion and tubulointerstitial fibrosis; these findings are closely related to the loss of renal function. Accumulating research suggests that oxidative stress plays a key role in the pathogenesis of diabetic nephropathy. In addition, antioxidant administration has been reported to have potentially beneficial effects in the human kidney and experimental diabetes.1-4
Oxidative stress has been suggested to play a role as a common mediator in apoptosis5,6 and in particular diabetic nephropathy, a state in which oxidative stress increased.1 Recent reports provide evidence that high ambient glucose can promote apoptosis in vitro, suggesting potential cellular damage as a result of hyperglycemia in diabetes in vitro.7 However, it is uncertain whether apoptosis occurs in the kidney during the course of diabetes.
Vitamin C plays a central role in the antioxidant defense system8 and apotosis.9 In the general absence of metal ion-catalyzed reactions, vitamin C is qualitatively the single most important plasma antioxidant. Thus, vitamin C is the antioxidant of choice in antioxidant experiments in vitro.10 However, its efficacy in clinical use remains to be confirmed.
Therefore we investigated the occurrence of apoptosis in the diabetic kidney and evaluated the effects of the antioxidant vitamin C on apoptosis, oxidative stress, and the functional and pathologic changes in the kidney of diabetic rats.
The Otsuka Long-Evans Tokushima Fatty rats, an animal model of human type 2 diabetes, and Long-Evans Tokushima Otsuka rats (n = 8) as a control strain were supplied by Otsuka Pharmaceutical, Tokushima, Japan. The characteristic features of OLETF rats are known as a gradual and late onset of hyperglycemia, a chronic course of disease, mild obesity, and a typical renal pathologic finding similar to that of humans.11 Male diabetic rats weighing 280 - 320g at 12 weeks of age were randomized into an untreated diabetic group (n = 8) and a vitamin C-treated diabetic group (n = 8). The research protocol was approved by the Yonsei University Wonju College of Medicine's animal ethical committee. Treatment was started at 16 weeks of age. The untreated diabetic group was given untreated drinking water. The vitamin C-treated diabetic group was given 10 g/L vitamin C (Ascorbic acid®) in their drinking water. Diabetic rats receiving vitamin C supplementation consumed an average of 0.9g/kg of body weight/day. Throughout the study all rats were maintained on standard rat chow (EP2®, Sam Yang, Seoul, Korea). Body weights and plasma glucose concentrations were determined at 8-week intervals. All rats were killed at 32 weeks of age. Blood was collected from the abdominal aorta, and serum was frozen to determine protein, albumin and creatinine excretion. The kidneys were perfused so as to be free of blood with PBS, and a portion was fixed in 4% paraformaldehyde for pathologic evaluation. A second portion was quick-frozen in liquid nitrogen to measure malondialdehyde (MDA).
Twenty-four-hour urine samples were obtained from animals in metabolic cages with access to drinking water only at 8-week intervals. Urinary total protein excretion was measured by the quantitative 3% sulfosalycylic acid technique.12 Urinary albumin excretion was measured by a quantitative reaction with bromocresol green.13 All samples were assayed in triplicate and a mean value was calculated for each rat.
One hundred milligrams of kidney was placed in 1 mL of 10 mM PBS (pH 7.0) containing 50 µM butylated hydroxytoluene and homogenized with 10 strokes of a rotary homogenizer driven at 20 rev/min. The suspension was centrifuged at 10,000g for 10 min and the supernatant used to determine MDA and protein. MDA was determined as described earlier14 using thiobarbituric acid as a standard and was prepared at concentrations in the range of the 0.2 to 2.5 µM/L. Supernatant or the standard (250 µL) was added to 250 µL of 10% trichloroacetic acid and placed in a boiling water bath for 2 min, after which 500 µL of 0.67% thiobarbituric acid was added and the tubes were again incubated in the bath for 10 min. The tubes were cooled and centrifuged at 10,000g for 3 min, and the supernatant was read at 535 nm using a Gilford spectrophotometer. The amount of MDA formed was determined by spectrofluorometry (SPF-500C, SLM Instruments) at an emission wavelength of 553 nm, then calculated using a tetraethoxypropane standard curve.
Random sections (3 µm thick) of the renal cortex were stained with periodic acid-Schiff (PAS). The surface areas of 100 glomeruli were measured with the Image-Pro (Version 1.2; Media Cybernetics, USA) software program on PAS stained tissue section and glomerular volume (VG) was calculated according to the method of Weibel and Gomez.15 VG=Area1.5 × 1.38/1.01 where 1.38 represents the shape coefficient, and 1.01 represents the size distribution coefficient. The mesangial matrix fraction was determined by the presence of PAS positive material present in the mesangial region, where researchers made sure to exclude cellular elements. Regarding the glomerular histopathologic changes, mesangial lesions were scored semiquantitatively by mesangial expansion and mesangial sclerosis. The scoring was performed by a blinded observer. Mesangial expansion was graded on a seven point scale: 0, normal; 1, mild segmental mesangial expansion; 2, mild diffuse mesangial expansion; 3, moderate diffuse mesangial expansion; 4, severe diffuse mesangial expansion; 5, segmental mesangial sclerosis; and 6, diffuse mesangial sclerosis.16 Interstitial fibrosis was scored semiquantitatively by a blinded observer who examined cortical tubulointerstitial fields on PAS-stained renal biopsies under a × 100 magnified field. At minimum, 30 fields were assessed in each biopsy. The following semiquantitative scores were used: 0 normal interstitum and tubules; 1, mild fibrosis with minimal interstitial thickening between the tubules; 2, modest fibrosis with moderate interstitial thickening between the tubules; 3, severe fibrosis with severe interstitial thickening between the tuules.17
Intranucleosomal DNA fragmentation was labeled in situ using an apoptosis detection system (DAKO, Baar, Switzerland) or the In Situ Cell Death Detection Kit (Roche, Basel, Switzerland). The formalin-fixed tissues were processed for paraffin embedding, and 4 µm-thick sections were cut and mounted on slides. After deparaffinization and rehydration, sections were digested with proteinase K and treated according to the protocol provided with the kit. Residues of digoxigenin-nucleotide were catalytically added to the 3'-OH ends of DNA by terminal deoxynucleotidyl transferase (TdT). Sections were then reacted with anti-digoxigenin antibody conjugated with peroxidase as a reporter enzyme. Diaminobenzidine for the apoptosis detection system or NBT/BCIP (Roche, Basel, Switzerland) for the In Situ Cell Death Detection Kit was used as the chromogenic substrate for peroxidase, producing a brown or deep blue reaction product that marked the nuclei of apoptotic cells. Apoptosis positive cells were counted in 6 consecutive interstitial fields under the microscope (× 200) and expressed as the mean number of positive stained cells in the interstitial field.
A separate portion of the kidney was also prepared for electron microscopy. Cubes were obtained from the renal cortex and these sections were fixed in 3% glutaraldehyde in 0.1M cacodylate buffer solution (pH 7.4) and postfixed in 1% osmium tetraoxide in the same buffer at 4℃. They were then dehydrated in a graded series of ethanol, passed through OY-1 (n-butyl glycidyl ether) and finally embedded in epoxyresin. Ultrathin sections were stained with uranyl acetate and lead citrate and were examined under a JEM 1200 EXII electron microscope (JEOL, Tokyo, Japan). Glomerular basement membrane (GBM) thickness was measured by the orthogonal intercept method in electron photomicrographs. On each glomerulus, 50 measurements were performed. Electron micrographs of glomeruli were taken with a magnification of × 10,000 without any bias. The slit pore density was represented as the number of slit pores per millimeter length of GBM according to published methods.18 The numbers of slit pores between the podocyte foot processes on the GBM were counted, and the slit pore density was determined by dividing the number of slit pores by the length of GBM measured.
As summarized in Tables 1 and 2, diabetic rats gained body weight continuously compared with the control rats. The mean weight was significantly higher in diabetic rats than in control rats during the study. Blood glucose concentrations were also significantly higher in the diabetic rats. The absolute mean kidney weight and kidney weight, expressed as a function of body weight of diabetic rats were significantly higher than that of control rats.
Vitamin C treatment did not affect blood glucose concentration, absolute kidney weight, or kidney weight expressed as a function of body weight in diabetic rats. Although the mean weight was slightly lower in vitamin C-treated diabetic rats when compared with the untreated diabetic rats at 24 weeks of age, there were no statistically significant differences between the two groups at other times.
The administration of vitamin C for 16 weeks significantly ameliorated proteinuria and albuminuria in diabetic rats, although proteinuria in vitamin C-treated diabetic rats remained statistically higher than in the untreated diabetic rats (Fig. 1).
Vitamin C treatment also significantly suppressed renal MDA in diabetic rats without significantly affecting blood glucose (Fig. 2).
The mean glomerular volume of diabetic rats was significantly higher than that of control rats. Also the mean thickness of the GBM in diabetic rats appeared to be higher than that of control rats, but this difference did not reach statistical significance (Table 2).
Semiquantitative analyses for mesangial and tubulointerstitial lesions from different experimental groups are summarized in Table 3. Glomerular expansion or sclerosis was significantly increased in diabetic rats when compared with control rats. Tubulointerstitial fibrosis was also markedly increased in diabetic rats compared with control rats. Vitamin C treatment effectively inhibited diabetes-associated glomerular and tubulointerstitial lesions.
The slit pore density (number of slit pores per underlying GBM length) as assessed by electron microscopy was decreased more in diabetic rats than control rats (Fig. 3). But this change was significantly restored by treatment with vitamin C (Fig. 3), associated with amelioration of albuminuria and proteinuria (Fig. 1).
Apoptosis occured in the tubular epithelial cells of proximal tubules in normal and diabetic rats. However, there were no apoptotic cells in the glomeruli in normal and diabetic rats (Fig. 4). The number of apoptotic cells was significantly decreased in vitamin C-treated diabetic rats when compared to the untreated diabetic rats (Fig. 5).
The present study demonstrates that vitamin C treatment, at a dose that reduces renal MDA accumulation, significantly ameliorates the progression of proteinuria and albuminuria. Vitamin C also decreases slit pore density, apoptosis of proximal tubular epithelial cells, degrees of glomerular injury, and tubulointerstitial damage in diabetic rats without significantly affecting plasma glucose.
Oxidative stress has been suggested to play an important role in the pathogenesis of diabetic nephropathy. Diabetic nephropathy is a state in which oxidative stress increases and antioxidant status is reduced, as has been documented.1 Lipid peroxidation increases in the kidney of diabetic animals; this might be due to decreases in antioxidant vitamins and enzymes. Glomerular O2-production was increased in untreated diabetic rats compared with control rats.19 The renal MDA level, an index of oxidative stress, was increased in the type 2 diabetic rat model20 and the results of present study are similar to those of prior reports on studies of a type 1 streptozotoc-ininduced diabetic rat model.
Recent reports provide evidence that high ambient glucose can promote apoptosis, suggesting potential cellular damage by hyperglycemia in diabetes.7,11 Hyperglycemia can trigger apoptosis in renal cells in vitro.7,21 High ambient glucose can induce DNA fragmentation22 and stimulate expression of apoptosis-regulatory genes23 in renal proximal tubular epithelial cells. In addition, apoptosis was documented in renal tubular epithelial cells; this may contribute to atrophy of the tubular epithelium and tubulointerstitial fibrosis in diabetic nephropathy. Diabetic nephropathy is characterized by an early period of renal growth with glomerular and tubular cell hypertrophy, but this is followed by progressive glomerulosclerosis and tubulointerstitial fibrosis, associated with loss of renal tissue. We studied whether apoptotic cell death occurs in diabetic nephropathy. We observed that apoptotic cells were selectively present in the epithelial cells of the proximal tubules. There were no apoptotic cells in the glomeruli of diabetic rats in this study. These findings are compatible with the conclusion that apoptosis was present, either in epithelial cells of the proximal or distal tubules, in endothelial cells or interstitial cells. In addition, no apoptosis was detected in human glomeruli cells. The present study, along with other investigations, provides evidence for the presence of apoptosis in the human diabetic kidney; this suggests a role for apoptosis in the gradual loss of renal masses.24
Oxidative stress is produced as a result of diabetic conditions and possibly causes a variety of tissue damage in patients with diabetes. Increased oxidative stress in the diabetic kidney may induce apoptosis, which may contribute to the development of diabetic nephropathy.25,26 The decrease in antioxidant defense, such as vitamin C, was also observed in patients with diabetic nephropathy.27 Diabetic nephropathy is characterized by mesangial expansion as well as glomerular and tubulointerstitial fibrosis. Thus, cell loss via apoptosis could be an important source for glomerular and tubulointerstitial sclerosis in diabetic nephropathy.
The effects of several antioxidants administered at the onset of experimental diabetes have been reported to prevent diabetic renal injury.28,29 Antioxidant therapy may be beneficial in preventing the development of diabetic nephropathy. Antioxidants might inhibit the development of diabetic nephropathy by suppressing apoptosis. The number of apoptotic cells was significantly decreased in vitamin C-treated diabetic rats when compared with untreated diabetic rats in this study. Vitamin C plays a central role in the antioxidant defense system. Vitamin C has been shown to protect all classes of lipids from oxidation under a number of relevant types of oxidant stress, while other non-enzymatic antioxidants such as vitamin A, vitamin E, glutathione, bilirubin, and urate merely lower the rate of oxidation or act in a more restricted, local environment.30 The uncharged form of vitamin C, dehydroascorbate, enters cells via a glucose transporter and is then converted back to ascorbate within these cells. Because dehydroascorbate and glucose compete for glucose transporters, the presence of hyperglycemia would work to exclude vitamin C from the cell and results in a decreased antioxidant capacity in some cell types that are dehydroascorbate-dependent such as renal tubular epithelial cells. In diabetes, vitamin C exclusion from tubular epithelial cells, through competition of glucose and dehydroascorbate for a common transport mechanism, will deprive the cells of antioxidant ability and could lead to reactive oxygen species accumulation.8 Dietary vitamins C and E with moderate exercise can strengthen the antioxidant defense system through the reduction of the reactive oxygen species and blood glucose levels.19 Treatment with vitamin C and desferrioxamine significantly decreased glomerular O2- production.19 Both vitamin C and vitamin E decreased lipid peroxidation and augmented the activities of antioxidant enzymes studied in diabetic rat kidneys as well as reduced urinary albumin excretion, decreased kidney weight and glomerular basement membrane thickness. These results indicate the potential utility of antioxidant vitamins in protecting against the development of diabetic nephropathy.20 Several other antioxidants such as taurine and other peroxynitrite scavengers attenuate hyperglycemia-induced apoptosis in renal tubular cells by inhibiting oxidative stress.7,21
Podocytes play an important role in the maintenance of normal glomerular permselectivity. Podocyte injury may lead to abnormal glomerular permeability and to structural alterations of GBM integrity, thereby leading to albuminuria and proteinuria. Further podocyte damage may finally result in glomerulosclerosis. Podocyte detachment is seen in a wide variety of glomerular diseases including metabolic or immunological glomerular injury, such as diabetic nephropathy.31 Recent evidence shows that in early diabetes, the podocyte suffers injury, dies, separates from the GBM and leaves areas of the GBM denuded or the podocytes, that are over-stretched, broaden their foot processes and try to cover the space.32 This stud revealed the slit pore density was noticeably reduced in the diabetic rats and was ameliorated by the blockade of oxidative stress.
In this study, the levels of urinary protein, albumin excretion, and renal MDA in vitamin C-treated diabetic rats is significantly lower than that of the untreated diabetic rats, but still statistically higher than the control rats. Urinary albumin excretion in our diabetic rats is similar to those reported in a previous study.18 Vitamin C may prove to be a new therapeutic agent for treating diabetic nephropathy. Vitamin C treatment suppressed apoptosis in renal proximal tubular epithelial cells without changing the blood glucose levels, supporting the hypothesis that apoptosis induced by oxidative stress causes reduction of renal function in chronic hyperglycemia. Vitamin C treatment also decreased the amounts of proteinuria and albuminuria. Our results suggest that vitamin C treatment can exert beneficial effects in diabetes patients, preserving in vivo renal function and structure. Our findings also support the potential usefulness of antioxidants in the treatment of diabetic nephropathy and further implicate oxidative stress in renal tubular cells apoptosis in diabetes.33 However, because our study is an animal study, these findings must be confirmed after further study, such as clinical trial, on human subjects.
In conclusion, treatment with vitamin C significantly suppressed the progression of renal injury in diabetic rats.
Figures and Tables
 | Fig. 1Effects of vitamin C on urinary protein and albumin excretion in diabetic rats. Values are means ± SD. D, diabetic rats; D + VC, diabetic rats treated with vitamin C; C, non-diabetic control rats. *p < 0.001 compared with C, †p < 0.001 compared with D, ††p < 0.05 compared with D. |
 | Fig. 2Effect of vitamin C on renal MDA in diabetic rats. Values are means ± SD. Values are means ± SD. D, diabetic rats; D + VC, diabetic rats treated with vitamin C; C, non-diabetic control rats. *p < 0.001 compared with C, †p < 0.001 compared with D. |
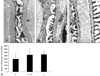 | Fig. 3Effect of vitamin C on slit pore density in diabetic rats. The slit pore density was expressed as the number of slit pores between podocyte foot processes per millimeter length of glomerular basement membrane by electron microscopy. Representative electron photomicrographs show examples of slit pores (arrows), which are more prevalent in the diabetic rats. D, diabetic rats (A); D + VC, diabetic rats treated with vitamin C (B); C, non-diabetic control rats(C). Magnification, × 40,000. **p < 0.05 compared with C, ††p < 0.01 compared with D. |
 | Fig. 4Apoptosis in diabetic glomeruli. Intranucleosomal DNA fragmentation was labeled using the In Situ Cell Death Detection Kit after 16 weeks of vitamin C treatment, as described in Materials and Methods. D, diabetic rats (A); C, non-diabetic control rats (B). Magnification, × 400. |
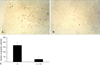 | Fig. 5Effects of vitamin C on apoptosis in diabetic rats. Intranucleosomal DNA fragmentation was labeled using the Apoptosis detection system after 16 weeks of vitamin C treatment, as described in Materials and Methods. D, diabetic rats (A); D + VC, diabetic rats treated with vitamin C (B). Magnification, × 100. Apoptosis positive cells were counted in sixconsecutive interstitial fields under the microscope and expressed as the mean numbers of positive cells of the interstitial field (C). Values are means ± SD. D, diabetic rats; D + VC, diabetic rats treated with vitamin C. *p < 0.05 compared with D. |
References
1. Horie K, Miyata T, Maeda K, Miyata S, Sugiyama S, Sakai H, et al. Immunohistochemical colocalization of glycoxidation products and lipid peroxidation products in diabetic renal glomerular lesions. Implication for glycoxidative stress in the pathogenesis of diabetic nephropathy. J Clin Invest. 1997. 100:2995–3004.

2. Trachtman H, Futterweit S, Maesaka J, Ma C, Valderrama E, Fuchs A, et al. Taurine ameliorates chronic streptozocin-induced diabetic nephropathy in rats. Am J Physiol. 1995. 269:F429–F438.

3. Craven PA, DeRubertis FR, Kagan VE, Melhem M, Studer RK. Effects of supplementation with vitamin C or E on albuminuria, glomerular TGF-beta, and glomerular size in diabetes. J Am Soc Nephrol. 1997. 8:1405–1414.

4. Bursell SE, Clermont AC, Aiello LP, Aiello LM, Schlossman DK, Feener EP, et al. High-dose vitamin E supplementation normalizes retinal blood flow and creatinine clearance in patients with type 1 diabetes. Diabetes Care. 1999. 22:1245–1251.

5. Buttke TM, Sandstrom PA. Oxidative stress as a mediator of apoptosis. Immunol Today. 1994. 15:7–10.

6. Jee SH, Kim HJ, Lee J. Obesity insulin resistance and cancer risk. Yonsei Med J. 2005. 46:449–455.

7. Allen DA, Harwood S, Varagunam M, Raftery MJ, Yaqoob MM. High glucose-induced oxidative stress causes apoptosis in proximal tubular epithelial cells and is mediated by multiple caspases. FASEB J. 2003. 17:908–910.

8. Chen L, Jia RH, Qiu CJ, Ding G. Hyperglycemia inhibits the uptake of dehydroascorbate in tubular epithelial cell. Am J Nephrol. 2005. 25:459–465.

9. Serbecic N, Beutelspacher SC. Vitamins inhibit oxidant-induced apoptosis of corneal endothelial cells. Jpn J Ophthalmol. 2005. 49:355–362.

10. Kang SA, Jang YJ, Park H. In vivo dual effects of vitamin C on paraquat-induced lung damage: dependence on released metals from the damaged tissue. Free Radic Res. 1998. 28:93–107.
11. Kawano K, Hirashima T, Mori S, Saitoh Y, Kurosumi M, Natori T. Spontaneous long-term hyperglycemic rat with diabetic complications. Otsuka Long-Evans Tokushima Fatty (OLETF) strain. Diabetes. 1992. 41:1422–1428.

12. Bradley M, Schumann GB, Ward PCJ. Henry JB, editor. Examination of urine. Clinical Diagnosis and Management by Laboratory Methods. 1979. Philadelphia: WB Saunders;559–634.
13. Rasanayagam LJ, Lim KL, Beng CG, Lau KS. Measurement of urine albumin using bromocresol green. Clin Chim Acta. 1973. 44:53–57.

14. Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979. 95:351–358.

15. Lane PH, Steffes MW, Mauer SM. Estimation of glomerular volume: a comparison of four methods. Kidney Int. 1992. 141:1085–1089.

16. Fukuzawa Y, Watanabe Y, Inaguma D, Hotta N. Evaluation of glomerular lesion and abnormal urinary findings in OLETF rats resulting from a long-term diabetic state. J Lab Clin Med. 1996. 128:568–578.

17. Thomas SE, Andoh TF, Pichler RH, Shankland SJ, Couser WG, Bennett WM, et al. Accelerated apoptosis characterizes cyclosporine-associated interstitial fibrosis. Kidney Int. 1998. 53:897–908.

18. Lahdenkari AT, Lounatmaa K, Patrakka J, Holmberg C, Wartiovaara J, Kestilä M, et al. Podocytes are firmly attached to glomerular basement membrane in kidneys with heavy proteinuria. J Am Soc Nephrol. 2004. 15:2611–2618.

19. Iino K, Iwase M, Sonoki K, Yoshinari M, Iida M. Combination treatment of vitamin C and desferrioxamine suppresses glomerular superoxide and prostaglandin E production in diabetic rats. Diabetes Obes Metab. 2005. 7:106–109.

20. Kedziora-Kornatowska K, Szram S, Kornatowski T, Szadujkis-Szadurski L, Kedziora J, Bartosz G. Effect of vitamin E and vitamin C supplementation on antioxidative state and renal glomerular basement membrane thickness in diabetic kidney. Nephron Exp Nephrol. 2003. 95:e134–e143.

21. Verzola D, Bertolotto MB, Villaggio B, Ottonello L, Dallegri F, Frumento G, et al. Taurine prevents apoptosis induced by high ambient glucose in human tubule renal cells. J Investig Med. 2002. 50:443–451.

22. Ishii N, Ogawa Z, Suzuki K, Numakami K, Saruta T, Itoh H. Glucose loading induces DNA fragmentation in rat proximal tubular cells. Metabolism. 1996. 45:1348–1353.

23. Ortiz A, Ziyadeh FN, Neilson EG. Expression of apoptosis-regulatory genes in renal proximal tubular epithelial cells exposed to high ambient glucose and in diabetic kidneys. J Investig Med. 1997. 45:50–56.
24. Kumar D, Robertson S, Burns KD. Evidence of apoptosis in human diabetic kidney. Mol Cell Biochem. 2004. 259:67–70.

25. Zhang W, Khanna P, Chan LL, Campbell G, Ansari NH. Diabetes-induced apoptosis in rat kidney. Biochem Mol Med. 1997. 61:58–62.

26. Murata I, Takemura G, Asano K, Sano H, Fujisawa K, Kagawa T, et al. Apoptotic cell loss following cell proliferation in renal glomeruli of Otsuka Long-Evans Tokushima Fatty rats, a model of human type 2 diabetes. Am J Nephrol. 2002. 22:587–595.

27. Hirsch IB, Atchley DH, Tsai E, Labbé RF, Chait A. Ascorbic acid clearance in diabetic nephropathy. J Diabetes Complications. 1998. 12:259–263.

28. Lal MA, Körner A, Matsuo Y, Zelenin S, Cheng SX, Jaremko G, et al. Combined antioxidant and COMT inhibitor treatment reverses renal abnormalities in diabetic rats. Diabetes. 2000. 49:1381–1389.

29. Melhem MF, Craven PA, Derubertis FR. Effects of dietary supplementation of alpha-lipoic acid on early glomerular injury in diabetes mellitus. J Am Soc Nephrol. 2001. 12:124–133.

30. Cao G, Booth SL, Sadowski JA, Prior RL. Increases in human plasma antioxidant capacity after consumption of controlled diets high in fruit and vegetables. Am J Clin Nutr. 1998. 68:1081–1087.

31. Stackhouse S, Miller PL, Park SK, Meyer TW. Reversal of glomerular hyperfiltration and renal hypertrophy by blood glucose normalization in diabetic rats. Diabetes. 1990. 39:989–995.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download