Abstract
Purpose
The arrhythmogenic effect of stem cells transplantation (SCT) in an infarct myocardium is still unknown. We investigated arrhythmogenicity of SCT in rat cryo-infarct model.
Materials and Methods
In rat cryo-infarct model, bone marrow mononuclear stem cells (MNSC, 1 × 107 cells) were transplanted into the infarct border zone (BZ) of the LV epicardium. We compared the optical mapping and inducibility of ventricular tachycardia/fibrillation (VT/VF) among normal (n = 5), cryo-infarct (n = 6), and SCT rats (n = 6).
Results
The VT/VF inducibility was higher in the cryoinfarct (47.2%, p = 0.001) and SCT groups (34.6%, p = 0.01) than in the normal group (12.8%). The induced VT/VF episodes persisted for more than 2 minutes in 4.3%, 26.4% and 17.3% in the normal, cryo-infarct and SCT group, respectively. In the SCT group, the action potential duration at 70% was shorter at the SCT site than the BZ during SR (75.2 ± 8.1 vs. 145.6 ± 4.4 ms, p = 0.001) and VT (78.2 ± 13.0 vs. 125.7 ± 21.0 ms, p = 0.001). Conduction block was observed at the SCT site and BZ during VT. However, no reentry or ectopic foci were observed around the SCT sites.
Modern reperfusion strategies and advances in the pharmacological management have resulted in an increasing proportion of patients with acute myocardial infarctions (AMIs) surviving with significant impairment of the systolic function of their left ventricles (LVs). On the basis of experimental studies that have suggested that the cardiac transfer of unfractionated bone marrow cells (BMCs) or bone marrow-derived stem and progenitor cells can promote functional improvements after an AMI,1-4 several clinical trials have explored the hypothesis that intracoronary infusion of autologous BMCs may enhance the recovery of the LV systolic function after an AMI.5-8 However, the arrhythmogenicity of stem cell transplantations (SCTs) still is unknown. Early studies by Smits et al.9 and Menasche et al.10,11 suggested there were proarrhythmic effects after stem cell therapy. In order for cell therapy to become widely applicable clinically, it has to be compatible both mechanically and electrically with the host myocardium. Although, it remains possible that these arrhythmias reflect the natural history of a myocardial infarction rather than the introduction of the new cells, it seems clear that we must consider the potential mechanisms of the arrhythmias and strategies to control or eliminate them.
In the present study, we investigated the electrical conduction of bone marrow mononuclear stem cells (MNSC) transplanted into the infarct border zone of rat LVs. We also evaluated the effect of transplanted MNSC on the inducibility of ventricular arrhythmias and whether or not they can be used as part of the reentry circuit or ectopic focus for arrhythmias.
All procedures were approved by the Institutional Animal Care and Use Committee and performed in accordance with the "Guidance for the Care and Use of Laboratory Animals" of Yonsei University College of Medicine.
Thirty adult Sprague-Dawley rats (weight 250 to 300 g) were used in this study as stem cell donors. The MNSCs were isolated from the femoral and tibial bones of donor rats. Extracted MNSCs from the donor rats were mixed with PBS (Dulbecco's Phosphate Buffered Saline, Gibco). The mixed solutions were centrifuged at 2000 rpm for 20 minutes and the buffy coat layer was extracted. This buffy coat was diluted with PBS and centrifuged at 1500 rpm for 5 minutes. After two centrifugations, the bone marrow MNSCs were extracted.
The rats were anesthetized with ketamine (75 mg/kg IP) and xylazine (5 mg/kg IP). After endotracheal intubation and mechanical ventilation (room air, rate 60 cycles/min, tidal volume 1 mL per 100 g of body weight, Harvard Apparatus Rodent Ventilator, model 683), the heart was exposed through a mid thoracotomy. The cryo-injury was created on the epicardial side of the LV of the rats. The first cryo-injury was made with a 1 cm cryo-injury rode (homemade) for 20 seconds and 4 additional cryo-injuries were then made for 60 seconds. The rats were allowed to recover under care.12
We started this study with 20 rats. Two rats died right after applying cryo-injury. And one rat died under normal raising facility 10 days after cryo-injury. So survival ratio was 85% for making this infarction model. Four weeks after the cryoinjury, the rats were reanesthetized and the chest was reopened for the injection of the MNSCs or saline and the infarcted region was visually identified by a mottled and pale appearance. Prior to cell transplantation, the bone marrow MNSCs were labeled with a fluorescent cell tracker [5-(and 6)-carboxyfluoresceindiacetate-succinimidyl-ester, CFDA, Molecular Probes, Eugene, OR] for Troponin I staining. Twenty mL of Saline (cryoinfarction group, n = 6) or the MNSCs with 20 mL of saline {stem cell transplantation (SCT) group, n = 6}, which contained about 1 × 107 cells, were injected directly into the border zone of the infarction with a 28-gauge needle attached to a 1 mL tuberculin syringe. Only one injection was performed at the left edge of cryo-infarcted area. When we exposed heart for MNSCs injection, we could clearly see the infarcted zone and easily identify the border zone. We counted bone marrow MNSCs for the each injection. A successful injection was typified by the formation of a bleb covering the infarct zone. Several rats without a cyo-infarct were used as a normal group (n = 5) to evaluate the normal electrical conduction and inducibility of ventricular tachycardia (VT) or fibrillation (VF).
Adult rat hearts (weight 250 to 300 g) were excised under general anesthesia at 4 weeks after the saline or stem cell injection. The ascending aorta was immediately cannulated and perfused at 15 to 20 mL/min with oxygenated Tyrode's solution (NaCl, 125.0; KCl, 4.5; MgCl2, 0.5; CaCl2 0.54; NaH2PO4, 1.2; NaHCO3 24.0; glucose, 5.5; albumin 50 mg/L, pH 7.4, 36.5℃). The heart was both perfused and superfused in a tissue bath made with transparent glass.
We paced the right ventricular epicardium of the Langendorff perfused hearts with a stimulus using a 2-ms pulse width and at twice the diastolic threshold current, using a programmed stimulator (Intermedics, Austin, TX, USA). VF was induced by burst pacing (PCL 75 ms, 5-ms pulse duration and 5-mA current for 5 seconds). The inducibility of the VT/VF was determined by the ratio of the successful VT/VF inductions to the number of burst pacing attempts (maximum 10). Once induced, VF was continuously monitored by a bipolar recording. Optical recordings were recorded immediately and every 2 minutes thereafter. If VF persisted for more than 5 minutes, it was terminated by defibrillation using 1 to 2 Joules. We compared the inducibility of the VT/VF and optical mapping among the normal (n = 5), cyo-infarction (n = 6), and SCT (n = 6) groups.
The optical mapping system has been described previously.13,14 The hearts were stained with di-4-ANEPPS (Molecular Probes, Inc,. Eugene, OR, USA). They were then excited with quasi-monochromatic light (500 ± 30 nm) from two green LED lamps (LL-50R30-G25, Optronix, Seoul, Korea). Fluorescent and scattered light was collected by an image-intensified charge-coupled device camera (CCD camera, Dalsa Inc., Waterloo, Canada). The data were gathered at 3.75-ms sampling intervals, acquiring from 100 × 100 sites simultaneously over a 35 × 35-mm2 area with a pixel size of 0.27 mm. The mapped area included parts of the right and left ventricular free walls. For detailed mapping of the anterior wall of the LV including the SCT site, we magnified the field to a 10 × 10-mm2 area. To decrease the motion related artifact, diacetyl monoxime (DAM) was used with an infusion rate of 8 mmol/l.13
The heart specimens were obtained from all rats. The specimens were fixed in 10% (v/v) buffered formaldehyde, dehydrated with a graded ethanol series, embedded in paraffin, and cut into 4-m-thick sections. The distribution µ of the cells was evaluated with a Hematoxyline & Eosin stain. The degree of fibrosis was evaluated with a Masson's trichrome stain. The slides were first examined at a 100 × magnification to identify the infarcted area, and then evaluated at a 400 × magnification.
For the detection of the CFDA-labeled cells and cardiac troponin I-positive cells, the tissue sections were stained immunofluorescently for cardiac troponin I using cyanine-conjugated anti-goat IgG secondary antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA, USA). The immunofluorescently stained sections were analyzed using a confocal microscope (LSM510, Carl Zeiss, Oberkochen, Germany).
The inducibility of VT/VF after stimulation was compared with a Chi-squire test between the three groups. Optical mapping data were processed through several image processing algorithms.13,14 After the data processing, pixels from the normal myocardium, border zone, STC site, and infarct site were chosen for optical signal analysis and APD determinations. The comparison of the APD duration between sinus rhythm and VT was made using the unpaired Student t-test. Differences with a value of p < 0.05 were considered statistically significant.
We used 180 ± 10 mg rat initially. The body weight was 235 ± 30, 240 ± 35, 238 ± 40 mg in normal, cryo-infarction, and SCT groups, respectively. The experiments lasted an average of 95 ± 25 minutes. After the excised hearts were perfused with Tyrode's solution and di-4-ANEPPS, a VT/VF induction study was performed 3 to 10 times in all hearts. Optical mapping was performed in all hearts after adequate staining with di-4-ANEPPS.
In the normal group, the depolarization during sinus rhythm was initiated from the mid-LV and spread to the rest of the ventricle without any conduction disturbances. The analysis of optical signals also revealed that the initiation of the depolarization was from the mid-LV (site 2) and then propagated in both directions (sites 1 and 2) (Figs. 2A, B and D). Multiple wavefronts meandered over the entire during VF with amorphorous optical signals (Figs. 2C and E).
In the cryo-infarction group, the depolarization during sinus rhythm was initiated from the mid-LV. However, the depolarization became delayed at the border zone, and almost disappeared at the cryo-infraction zone (Figs. 3A, B and D). During VF, multiple wavefronts appeared throughout the heart except for at the cryo-infarction zone (Figs. 3C and E). Conduction block was observed at the border zone and cryo-infarction zone (Fig. 3E).
In the SCT group, the depolarization also initiated from the mid-LV during sinus rhythm. The depolarization was delayed at the border zone, and almost disappeared at the cryo-infraction zone. However, the depolarization improved at the SCT sites (Figs. 4A, B and D). During the VT or VF, conduction block was observed at the border zone and infarct zone. Reentry around the entire infarction site was observed (Figs. 4C and E). However, no reentry or ectopic beats around the SCT site were observed. Conduction block was observed at the border and infarct zone.
In the normal group, VT or VF was induced in 6 of 47 burst pacing applications (12.8%) as compared with 25 of 53 in the cryo-infarction group (47.2%) and 18 of 52 in the SCT group (34.6%). Compared with the normal group, the VT/VF inducibility was higher in the cryo-infarction (p = 0.001) and SCT groups (Table 1, Fig. 1, p = 0.01). The VT/VF induced persisted for more than 2 minutes in 2 of 47 (4.3%), 14 of 53 (26.4%) and 9 of 52 stimulations (17.3%) in the normal, cryo-infarction and SCT groups, respectively.
The APD at 70% (ADP70) was analyzed during sinus rhythm and VT in the SCT group. The APD70 during sinus rhythm was 49.6 ± 2.0 ms, 75.2 ± 8.1 ms, and 145.6 ± 4.4 ms at the normal, stem cell transplant sites, and border zones, respectively. The APD70 in the SCT sites was shorter than that for the border zones (p = 0.001). The APD70 during VT was 72.7 ± 3.3 ms, 78.2 ±13.0 ms, and 125.7 ± 21.0 ms at the normal, stem cell transplant sites, and border zones, respectively. The APD70 of the SCT sites was also shorter than that of the border zone (Table 2, p = 0.001).
The microscopic appearance of a stem cell transplanted rat heart with a cryo-infarct is shown in Fig. 5. According to H & E staining (Figs. 5A and B) and Masson Trichrome staining (Figs. 5C and D), huge fibrosis was observed in the cryo-infarct sites from 1 to 6 o'clock in the LV, and the SCT sites exhibited less fibrosis with some cells (Figs. 5C and D). MNSCs transplanted in infarct border zone showed positive with troponin I staining (Fig. 6).
Heart failure affects an estimated 4.9 million people in the United States, with 550,000 new cases reported annually.15 Despite major improvements in medical therapy, a significant proportion of patients remain symptomatic. The disease processes leading to myocardial contractile dysfunction all, to a greater or lesser extent, occur in a patchy or regional pattern and, along with correspondingly heterogeneous electrical remodeling, create this nonuniformity associated with arrhythmogenesis. Emerging strategies of cell transplantation to restore myocardial function in the failing heart are focusing largely on the compelling goal of improving contractile function or promoting angiogenesis, with little attention given initially to how arrhythmic risk may be modified. One important question that this raises is whether this approach will suppress an arrhythmic tendency, by restoring greater uniformity of healthy tissue architecture and function, or whether it will further add to the heterogeneity, thereby enhancing any arrhythmic tendency.16,17
In the study by Smits et al.1 one of the five patients had sustained episodes of ventricular tachycardia and required implantable cardioverterdefibrillator (ICD) placement. The investigators also describe a subsequent unpublished experience of two sudden deaths and three serious ventricular arrhythmias in eight additional patients. These data seem to correspond to the Menasche et al.2,3 experience in which 4 of 10 patients rezuired ICD implantation for ventricular arrhythmias after open chest autologous skeletal myoblast transplantation. Proarrhythmia after stem cell therapy might be attributed to one or more of the following reasons: 1) Heterogeneity of action potentials between the native and the transplanted stem cells; 2) intrinsic arrhythmic potential of injected cells; 3) increased nerve sprouting induced by stem cell injection; and 4) local injury or edema induced by intramyocardial injection.17
Four weeks after cryo-injury, infarction sites were easily identified because it showed white color compared normal tissue. For appropriate tracking of MNSCs injection site, only one injection was performed at the left edge of cryoinfarcted area. In our experience, MNSC injection site showed revascularization with reddish color after 4 weeks. Electrical conduction between cells was not evaluated in this study. This study evaluated so called electrical conduction between tissues with high resolution optical mapping system.
In the rat cryo-infarct model, the infarct lesion exhibited almost no electrical activity. The border zone exhibited a delayed depolarization and broad APD as compared to the normal myocardium. In this study, we could find that MNSCs transplanted at the border zone of a cryo-infarction improved the electrical conduction.
The data about the influence of a cryo-infarct on the electrical conduction is limited in the rat cryo-infarct model. However, data obtained from 4- to 5-day-old canine infarcts showed that the healing infarct undergoes structural and functional changes. The surviving epicardial cells overlying the infarct have abnormal action potentials with diminished upstrokes with the loss of the plateau and a shorter action potential duration. The density and kinetics of a number of ion channel become altered, and the sodium ion and calcium ion currents become reduced, as do the transient outward potassium ion currents and delayed and inward rectifying potassium ion currents.18 During this stage, reentrant VT in the so-called epicardial border zone (the layer of surviving cells overlying the infarct) can easily be induced by premature stimuli. Both the cellular abnormalities, as well as a redistribution of the intercellular gap junction, play a role in determining the reentry.19 These VTs may degenerate into VF, especially in the presence of a high sympathetic tone,20 but this is uncommon.
Over the next few weeks, transmembrane action potentials of the surviving cells gradually return to normal, as most of the ion channels recover, and by 2 months, the action potential configuration in both canine and human infarcts is completely normal.21,22 It is difficult to be certain when the healing phase of a myocardial infarction is over and when the fully healed phase begins. It is likely that the electrophysiological substrate for VT gradually develops over several weeks and remains stable from several months to 15 to 20 years.23
According to Zhang, et al.24 stem cells showed conduction disturbances in vitro patch-clamp studies. However, the border zone already exhibited a severe conduction disturbance and the transplanted MNSCs showed differentiation to troponin I positive cells and improved the electrical conduction in this study. The repolarization phase of the APD was significantly prolonged at the border zone. This prolonged APD may promote arrhythmias. The transplanted MNSCs also shorten the APD at the border zone. However, the limited method used in this study could not explain whether the improvement in the electrical conduction was caused by the regeneration of the myocardium or only by neovascularization.
The MNSCs transplantation did not effect the inducibility or duration of the VT/VF. The result was in concordance with the improved electrical conduction, especially for the repolarization by the MNSC transplantation. When we were considering the proarrhythmic effects of the MNSCs transplantation, the mechanism was thought to be possibly due to reentry caused by an electrical heterogeneity, or ectopic focus with automaticity or triggered activity. However, we could not find any evidence that the MNSC transplanted sites served as a part of a reentry circuit or ectopic focus. The severe prolongation of the repolarization and heterogeneity which were observed at the border zones, improved at the stem cell transplantation sites. That improvement may have attenuated the arrhythmogeneity of this border zone.
In conclusion, the electrical conduction was improved more by the MNSCs transplantation. The MNSCs transplantation in the rat cryo-infarction model did not increase the inducibility of the VT/VF. There was no evidence of augmentation of the arrhythmia from the MNSCs in the cryo-infarct rat model.
The type of infarct model used in this study was a cryo-infarction model. Coronary artery ligation model is more popular for the evaluation of infarct model. Actually we also studied with ligation model initially. Compared to ligation model, the cryo-infarction model is known to have less arrhythmias and ventricular aneurysms. However, the optical mapping in the LAD ligation model was not appropriate because of an unclear infarct border. In this study, only MNSCs were used for the stem cell transplantation. Therefore, further studies are needed to apply this result to other cell types. If we could have monitored rats ambulatory ECG, it would have been better for evaluation of arrhythmia.
Figures and Tables
Fig. 1
Comparison of the ventricular tachycardia or fibrillation (VT/VF) inducibility between the control, cryo-injury, and stem cell transplantation (SCT) groups. SCT indicates stem cell transplantation group. The inducibility of VT/VF was 12.8%, 34.6%, and 47.2% in control, cryoinjury, and stem cell transplantation group, respectively. Cryoinjury and STC group had higher VT/VF inducibility than control (p = 0.01).

Fig. 2
Optical mapping of the control rat hearts during normal sinus rhythm (B and D) and ventricular fibrillation (C and E). (A) Optical mapping was recorded from the epicardium of the left ventricle (box). The numbers 1 to 3 represent the sites where the optical signals were taken. (B and C) Pseudo color membrane voltage maps at certain times are presented. (D and E) Optical signals were recorded from sites 1 to 3. During sinus rhythm, the depolarization was initiated from the mid-LV (site 2) and spread to the rest of the heart (sites 1 and 3). During VF, multiple wavefronts meandered over the entire heart during VF with amorphorous optical signals recorded from sites 1 to 3.
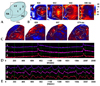
Fig. 3
Optical mapping of cryo-infarct rat hearts during normal sinus rhythm (B and D) and ventricular fibrillation (C and E). (A) Optical mapping was recorded from the epicardium of the LV (box). The numbers 1 to 4 represent the sites where optical signals were taken. Sites 1 and 2 are normal myocardium, 3 a border zone, and 4 a cryo-infarct. (B and C) Pseudo color membrane voltage maps at certain times are presented. Depolarization was initiated from the mid-LV (site 2), and spread to the normal myocardium (site 1, 544 ms) and then to the border zone (site 3,564 ms). (D and E) Optical signals recorded from sites 1 to 4. During sinus rhythm, depolarization was initiated from the mid-LV (site 2) and spread to the rest of the heart (sites 1 and 3). However, no depolarization was observed in the cryo-infarct (site 4). During VF, amorphorous waves are observed in the normal myocardium and border zone (sites 1 to 3) but not in the cryo-infarct (site 4).

Fig. 4
Optical mapping of a cryo-infarct in stem cell transplanted rat hearts during normal sinus rhythm (B and D) and ventricular fibrillation (C and E). (A) Optical mapping was recorded on the anterior LV including the SCT site (Box). The numbers 1 to 3 represent the sites where optical signals were recorded. (B and C) Pseudo color membrane voltage maps at certain times are presented. (D and E) Optical signals were recorded from sites 1 (normal zone), 2 (stem cell transplanted site), and 3 (border zone) as marked in the first sub-panel of panels B and C (asterisk). The ventricular tachycardia terminated spontaneously.
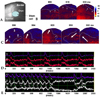
Fig. 5
Microscopic appearance of a cryo-infarct in a stem cell transplanted rat heart (Fig. 4). (A) H & E staining revealed cells in the mononuclear stem cell implant site. (B) Magnified view of the box in A. The stem cell transplant site had a higher density of cells (arrow). (C) With Masson Trichrome staining, a huge amount of fibrosis was observed from 1 to 6 o'clock along the left ventricle after the cryo-infarction (blue color). The stem cell transplant site had a brown color (box). (D) Magnified view of the box in C. The stem cell transplant site had less fibrosis (brown color, arrow).
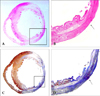
Fig. 6
Differentiation of transplanted MNSCs into cardiomyocytes. MNSCs transplanted in infarct border zone showed rich viable cells (blue color in A). Transplanted MNSCs were engrafted in the myocardium and stained for cardiac troponin I. Troponin I positive cells are presented with red color in B, green color in C, and yellow color in D.
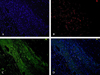
Table 1
Comparison of the Ventricular Tachycardia or Fibrillation (VT/VF) Inducibility between the Control, Cryo-Injury, and SCT Groups

Table 2
Comparison of the APD at 70% During Sinus Rhythm and Ventricular Tachycardia which was Presented in Figs. 4B and D, Respectively

References
1. Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001. 410:701–705.

2. Kamihata H, Matsubara H, Nishiue T, Fujiyama S, Tsutsumi Y, Ozono R, et al. Implantation of bone marrow mononuclear cells into ischemic myocardium enhances collateral perfusion and regional function via side supply of angioblasts, angiogenic ligands, and cytokines. Circulation. 2001. 104:1046–1052.

3. Balsam LB, Wagers AJ, Christensen JL, Kofidis T, Weissman IL, Robbins RC, et al. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature. 2004. 428:668–673.

4. Yoon YS, Wecker A, Heyd L, Park JS, Tkebuchava T, Kusano K, et al. Clonally expanded novel multipotent stem cells from human bone marrow regenerate myocardium after myocardial infarction. J Clin Invest. 2005. 115:326–338.

5. Strauer BE, Brehm M, Zeus T, Köstering M, Hernandez A, Sorg RV, et al. Repair of infarcted myocardium by autologous intracoronary mononuclear bone marrow cell transplantation in humans. Circulation. 2002. 106:1913–1918.

6. Assmus B, Schächinger V, Teupe C, Britten M, Lehmann R, Döbert N, et al. Transplantation of Progenitor Cells and Regeneration Enhancement in Acute Myocardial Infarction (TOPCARE-AMI). Circulation. 2002. 106:3009–3017.

7. Fernández-Avilés F, San Román JA, García-Frade J, Fernández ME, Peñarrubia MJ, de la Fuente L, et al. Experimental and clinical regenerative capability of human bone marrow cells after myocardial infarction. Circ Res. 2004. 95:742–748.

8. Wollert KC, Meyer GP, Lotz J, Ringes-Lichtenberg S, Lippolt P, Breidenbach C, et al. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet. 2004. 364:141–148.

9. Smits PC, van Geuns RJ, Poldermans D, Bountioukos M, Onderwater EE, Lee CH, et al. Catheter-based intramyocardial injection of autologous skeletal myoblasts as a primary treatment of ischemic heart failure: clinical experience with six-month follow-up. J Am Coll Cardiol. 2003. 42:2063–2069.

10. Menasché P, Hagège AA, Scorsin M, Pouzet B, Desnos M, Duboc D, et al. Myoblast transplantation for heart failure. Lancet. 2001. 357:279–280.

11. Menasché P, Hagège AA, Vilquin JT, Desnos M, Abergel E, Pouzet B, et al. Autologous skeletal myoblast transplantation for severe postinfarction left ventricular dysfunction. J Am Coll Cardiol. 2003. 41:1078–1083.

12. Ryu JH, Kim IK, Cho SW, Cho MC, Hwang KK, Piao H, et al. Implantation of bone marrow mononuclear cells using injectable fibrin matrix enhances neovascularization in infarcted myocardium. Biomaterials. 2005. 26:319–326.

13. Lee MH, Lin SF, Ohara T, Omichi C, Okuyama Y, Chudin E, et al. Effects of diacetyl monoxime and cytochalasin D on ventricular fibrillation in swine right ventricles. Am J Physiol Heart Circ Physiol. 2001. 280:H2689–H2696.

14. Garfinkel A, Kim YH, Voroshilovsky O, Qu Z, Kil JR, Lee MH, et al. Preventing ventricular fibrillation by flattening cardiac restitution. Proc Natl Acad Sci U S A. 2000. 97:6061–6066.

15. Hunt SA, Baker DW, Chin MH, Cinquegrani MP, Feldman AM, Francis GS, et al. ACC/AHA guidelines for the evaluation and management of chronic heart failure in the adult: executive summary: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1995 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the International Society for Heart and Lung Transplantation; endorsed by the Heart Failure Society of America. Circulation. 2001. 104:2996–3007.

16. Peters NS. Arrhythmias after cell transplantation for myocardial regeneration: natural history or result of the intervention? J Cardiovasc Electrophysiol. 2005. 16:1255–1257.

17. Makkar RR, Lill M, Chen PS. Stem cell therapy for myocardial repair: is it arrhythmogenic? J Am Coll Cardiol. 2003. 42:2070–2072.
18. Pinto JM, Boyden PA. Electrical remodeling in ischemia and infarction. Cardiovasc Res. 1999. 42:284–297.

19. Peters NS, Coromilas J, Severs NJ, Wit AL. Disturbed connexin 43 gap junction distribution correlates with the location of reentrant circuits in the epicardial border zone of healing canine infarcts that cause ventricular tachycardia. Circulation. 1997. 95:988–996.

20. Wit AL, Janse MJ. The ventricular arrhythmias of ischemia and infarction. Electrophysiological mechanisms. 1993. Mount Kisco, NY: Futura Publishing.
21. Ursell PC, Gardner PI, Albala A, Fenoglio JJ Jr, Wit AL. Structural and electrophysiological changes in the epicardial border zone of canine myocardial infarcts during infarct healing. Circ Res. 1985. 56:436–451.

22. de Bakker JM, van Capelle FJ, Janse MJ, Wilde AA, Coronel R, Becker AE, et al. Reentry as a cause of ventricular tachycardia in patients with chronic ischemic heart disease: electrophysiologic and anatomic correlation. Circulation. 1988. 77:589–606.

23. Callans DJ, Josephson ME. Zipes DP, Jalife J, editors. Ventricular tachycardia associated with coronary artery disease. Cardiac electrophysiology: From cell to Bedside. 1995. 2nd ed. Philadelphia: WB Saunders;732–743.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download