Abstract
Purpose
Cell cycle progression is regulated by interactions of specific cyclins and cyclin dependent kinases (CDKs) at the G1-S and G2-M checkpoints and cell cycle deregulation plays a major role in carcinogenesis of human cancers.
Patients and Methods
To investigate the role of cell cycle regulators in the pathogenesis and progression of human gastric cancers, 23 cases of gastric carcinomas were examined for the expression of cyclin B1, p34cdc2, p27Kip1 and p53 by immunohistochemical methods, and gene expression was correlated with various clinicopathologic findings.
Results
Out of 23 cases studied, cyclin B1 was diffusely expressed in 20 cases (87.0%), p34cdc2 in 14 cases (60.9%) and p53 in 12 cases (52.2%), whereas in normal gastric tissues, cyclin B1 and p34cdc2 were weakly expressed and p53 was not expressed. In contrast, p27Kip1 was expressed in only 8.7% of gastric carcinomas compared with 78.3% of normal gastric tissues. There was correlation between the expression of cyclin B1 and expression of p34cdc2 (p = 0.002), between the expression of cyclin B1 and loss of p27Kip1 (p = 0.025), and between the expression of p34cdc2 and loss of p27Kip1 (p = 0.065). In addition, expression of cyclin B1 was correlated with regional lymph node metastasis (p = 0.032).
Conclusion
Our results indicate that cyclin B1 and p34cdc2 are involved in the genesis or progression of gastric cancers. Furthermore, overexpression of cyclin B1 may play an important role in lymph node metastatic potential of gastric cancer. Thus, abnormal expression of cyclin B1 and CDKs, overexpression of p53 and loss of p27Kip1 expression may play important roles in human gastric carcinogenesis.
Cell growth is tightly controlled by interactions of cyclins, cyclin dependent kinases (CDKs), and CDK inhibitors (CDKIs). Cyclins are proteins that govern progression through key checkpoints in the cell cycle by binding and activating specific CDKs.1 Cyclin/CDK complexes stimulate cell cycle progression, while CDKIs induce cell cycle arrest by downregulating CDK activity.2,3 Dysregulated expression of these cell cycle-related molecules, such as overexpression of cyclins and CDKs, is closely linked to uncontrolled proliferation and malignant transformation of the cell.4
In eukaryotes, the cell cycle is coordinated by several protein kinases composed of a CDK subunit and the corresponding regulatory cyclin subunit. p34cdc2 is a protein kinase that functions in conjunction with cyclin B1 and CDKIs at the G2-M checkpoint to control mitosis.5 Alteration in cyclin B1/p34cdc2 is a widespread feature of tumorigenesis. Regulation of p34cdc2 activity is a complex process involving cyclin binding, subunit phosphorylation, CDK inhibitor binding, and cyclin degradation. The CDKIs identified in gastric cells are subdivided into two structurally unrelated classes, the INK family that includes p15INK4B/MTS2, p16INK4A/MTS1, and p18, and the KIP family that contains p27Kip1, p21WAF1/Cip1 and p57Kip2.6 The inhibitor p27Kip1 does not associate with kinase subunits unless a cyclin is present. In vitro, p27Kip1has broad specificity, inhibiting the kinase activity of G1 cyclin complexes (Cyclin A-cdk2, Cyclin D-cdk4 and Cyclin E-cdk2) and, to a lesser extent, the mitotic cyclin B-p34cdc2 complex. p27Kip1 regulation is an essential step in the pathway that links mitogenic signals to cell cycle progression.7
In addition to cyclins, CDKs and CDKIs, alterations in the tumor suppressor gene, p53, are frequently observed in human neoplasms.8
Gastric cancers are the one of the most common cancers in the Korean population. In this study, we examined the expression of cyclin B1, p34cdc2, p27Kip1 and p53 in 23 cases of gastric cancer by immunohistochemical methods and analyzed the correlation of expression of cyclin B1, p34cdc2, p27Kip1 and p53 with various clinicopathologic findings.
Twenty-three cases of gastric cancer that were histologically diagnosed from 1997 to 1998 at Eulji General Hospital were included in this study. Follow-up data of the cases were retrospectively retrieved from hospital clinical records and patients' families. Classification, diagnoses and staging of gastric cancers were based on the AJCC Cancer staging manual of American Joint Committee on Cancer.9
Paraffin-embedded, 4µm-thick tissue sections were prepared for immunohistochemistry. Slides were dehydrated, deparaffinated through xylene, and then rehydrated through graded alcohols. To retrieve antigenicity, the sections were heated in a microwave oven in 10mM citrate buffer at pH 6.0 for 30 min. Sections were then immersed in methanol containing 2% H2O2 for 30 min to block endogenous peroxidase activity and pretreated with normal goat serum to reduce nonspecific reactions. Sections were incubated at 4℃ overnight with each primary antibody. The primary antibodies used were: cyclin B1 (Novocastra, Newcastle, UK, 1:20), p34cdc2 (Santa Cruz, CA, USA, 1:100), p27Kip1 (Neo Markers, CA, USA, 1:50) and p53 (Neo Markers, CA, USA, 1:50). Immunohistochemical staining was performed using a standard streptavidin-biotin complex procedure. 3-amino-9-ethyl carbazole (AEC) was used as a substrate, and Meyer's hematoxylin was used for counterstaining. For gastric carcinomas, three representative areas were photographed by digital camera under × 400 magnification, and the total cell number and positively stained cells were counted to assess the expression of cyclin B1, p34cdc2, p27Kip1 and p53. Staining in more than 5% of the tumor cells was considered positive. For immunostaining of cyclin B1 and p34cdc2, cytoplasmic and/or nuclear staining was considered positive. For immunostaining of p27Kip1 and p53, nuclear staining was considered positive. For normal gastric tissue, three representative areas were photographed under × 400 magnification in glandular epithelial areas.
The relationship between expression of cyclin B1, p34cdc2, p27Kip1, p53 and various clinicopathological findings were evaluated using Fisher exact test and Pearson Chi-Square test. Kaplan-Meier survival curves were constructed to access whether levels of cyclin B1, p34cdc2, p27Kip1 and p53 had any effect on overall survival of patients with gastric cancer and the resulting curves were compared using the log-rank test. A p value less than 0.05 was considered statistically significant. Statistical analyses were performed with SPSS for Windows (version 8.0).
Clinical data of the patients with gastric cancer are summarized in Table 1. Fifteen cases were men and eight cases were women with a mean age of 57.1yrs (range 29 to 77 yrs). The median follow-up period was 68 months (range 3 months to 108 months). Patient survival was classified as no evidence of disease (NED, n = 15), death of disease (DOD, n = 5), and alive with disease (AWD, n = 3). There were four cases with stage I cancer, three cases with stage II, seven cases with stage IIIa, six cases with stage IIIb and three cases with stage IV. The histologic subtypes included tubular adenocarcinoma (TA, n = 13) and signet ring cell carcinoma (SRCC, n = 10).
Out of 23 cases, cyclin B1, p34cdc2 and p53 were diffusely expressed in 20 cases (87.0%), 14 cases (60.9%) and 12 cases (52.2%), respectively (Fig. 1). The mean labeling indices of cyclin B1 and p34cdc2 were 27.7% and 40.1%, respectively. Immunostaining of cyclin B1 and p34cdc2 was detected predominantly in the cytoplasm. In normal gastric tissue, cyclin B1 and p34cdc2 were weakly expressed in the cytoplasm: the mean labeling indices of cyclin B1 and p34cdc2 in the glandular epithelial cells of normal gastric tissues were 5% and 15%, respectively. Immunostaining of p53 was detected predominantly in the nuclei. The expression rate of p27Kip1 was 8.7% in gastric carcinomas and 78.3% in normal gastric tissues, indicating loss of p27Kip1 expression in gastric cancer (Fig. 2). The expression of cyclin B1 was correlated with regional lymph node metastasis (p = 0.032) (Table 2).
Survival analysis was performed in 23 patients with mean observation duration of 78.6 months. Expression of cyclin B1, p34cdc2, p27Kip1 and p53 showed no significant influence on overall survival, regardless of the cut-off value in the Kaplan-Meier survival curves (Fig. 3).
Progression through the cell cycle is controlled by a series of cyclins, CDKs and CDKIs. To evaluate the role of cyclin B1/p34cdc2 in the pathogenesis of gastric carcinoma, and to determine whether over-expression of cyclin B1/p34cdc2 influences the prognosis including overall survival, we studied expression of cyclin B1 and p34cdc2 in 23 patients with gastric carcinoma.
In this immunohistochemical study, the mean labeling indices of cyclin B1 and p34cdc2 in gastric carcinomas were 27.7% and 40.1%, respectively. In contrast, the mean labeling indices of cyclin B1 and p34cdc2 in the glandular epithelial cells of normal gastric tissues were 5% and 15%, respectively, much lower than in gastric carcinomas. Furthermore, the staining patterns between gastric carcinomas and normal gastric tissues were quite different. In normal gastric tissue, the staining pattern indicated multifocal expression of cyclin B1 and p34cdc2, whereas diffuse staining was observed in gastric carcinomas. These results suggest that cyclin B1 and p34cdc2 may play a role in the genesis or progression of gastric carcinomas, although the mechanism by which cyclin B1 and p34cdc2 participate in tumor progression remains unclear. p53 is known to play a role in arrest at the G1 checkpoint through p53-mediated synthesis of the cell cycle inhibitor p21, and recent studies have revealed that p53 also regulates the G2 checkpoint by inactivating p34cdc2 kinase. The inactivation of p34cdc2 kinase results, at least in part, from repression of cyclin B1 and p34cdc2 transcription. However, constitutive expression of both cyclin B1 and p34cdc2 can override p53-mediated G2-M arrest. Mutations in p53 have been found in a variety of malignancies;10,11 this p53 dysregulation may result in a failure to repress cyclin B1 and p34cdc2, leading to overexpression of cyclin B1/p34cdc2 and G2-M transition without the G2 checkpoint.
Overexpression of cyclin B1 may be caused by several mechanisms, including impaired proteolytic degradation or uncontrolled protein synthesis. At present, it is not clear how overexpression of cyclin B1 and p34cdc2 influences oncogenesis and tumor progression. Cyclin B1 is normally present at high levels in the later cell cycle phases and is re-synthesized as late as the beginning of S phase, whereas p34cdc2 is expressed during all phases of the cell cycle except G0.11 This may explain why the labeling index of p34cdc2 is higher than that of cyclin B1 in both normal gastric tissue and gastric cancer. Although overexpression of cyclin B1 has been shown to be an important factor affecting survival in several malignant diseases including esophageal squamous cell carcinoma,12 non-small cell carcinoma,13 and hepatocellular carcinoma,14 in this study the expression level of cyclin B1 and p34cdc2 did not influence gastric cancer survival rates according to Kaplan-Meier survival curve analysis.
To gain a better understanding of the molecular changes underlying the potentially aggressive behavior of gastric carcinomas, we studied immunoreactivity for p53 and p27Kip1 in gastric carcinomas. Alterations in growth control pathways play an essential role in the origin and development of neoplasms; in particular, disruption of the tumor suppressor gene p53 has been implicated as the main mechanism leading to the loss of cell-cycle control in human malignancies.12,15,16 Mutations in the p53 gene, located on the short arm of chromosome 17, are the most common genetic lesions observed in human neoplasms,8,9,17,18 and the loss of wild-type p53 has been reported in gastric carcinomas of the stomach.19,20 This dysfunction is generally associated with increased immunostaining for p53 protein. On the other hand, some neoplasms exhibit down-regulation of the cyclin-dependent kinase inhibitor p27Kip1, a change usually accompanied with a loss of p27Kip1 immunoreactivity and aggressive tumor behavior. p27Kip1 is a CDK inhibitor that regulates cell proliferation by binding and inhibiting G1 cyclin- CDK complexes, preventing progression through the G1 and S phases of the cell cycle.21,22 The high levels of p27Kip1 found in normal gastric epithelial cells suggest that its role may be to keep cells in the quiescent phase. Loss of p27Kip1 protein expression may result in cellular proliferation, tumor development and progression. Many studies have characterized p27Kip1 as an independent prognostic factor in various human cancers, including gastric carcinomas.6,7,22 Moreover, p27Kip1 appears to promote apoptosis in human gastric carcinoma cells and may be instrumental in the regression of gastric carcinomas through unknown mechanisms.
Future studies are required to better delineate the effectors that mediate the malignant phenotype, and studies such as ours on gene expression in human cancer samples provide essential information to improve our understanding of the mechanisms regulating gastric carcinogenesis.
Figures and Tables
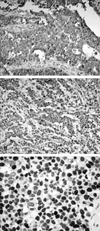 | Fig. 1 Immunostaining for cyclin B1 (A), p34cdc2 (B) and p53 (C) in gastric carcinoma (× 400). Both cyclin B1 (A) and p34cdc2 (B) are expressed in the cytoplasm and nuclei of carcinoma cells. p53 (C) is strongly expressed in nuclei of carcinoma cells. |
 | Fig. 2 Immunostaining for p27Kip1 in normal gastric tissue (A) and gastric carcinoma (B) (× 400). p27Kip1 is strongly expressed in the nuclei of normal gastric cells (A), and weakly expressed in the nuclei of carcinoma cells (B). |
 | Fig. 3Kaplan-Meier survival curve stratified according to the expression level of cyclin B1, p34cdc2, p27Kip1 and p53. Expression of cyclin B1 was stratified as ≥ 5% (n = 20) and < 5% (n = 3), (A); expression of p34cdc2 was stratified as ≥ 5% (n = 14) and < 5% (n = 9), (B); expression of p27Kip1 was stratified as ≥ 5% (n = 2) and < 5% (n = 21), (C); and expression of p53 was stratified ≥ 5% (n = 12) and < 5% (n = 11), (D). Overall survival was not significantly influenced by expression of cyclin B1, p34cdc2, p27Kip1 or p53. |
References
3. Sherr CJ, Roberts JM. Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev. 1995. 9:1149–1163.

5. Ohta T, Okamoto K, Isohashi F, Shibata K, Fukuda M, Yamaguchi S, et al. T-loop deletion of CDC2 from breast cancer tissues eliminates binding to cyclin B1 and cyclin-dependent kinase inhibitor p21. Cancer Res. 1998. 58:1095–1098.
6. Nakayama K, Nakayama K. Cip/Kip cyclin-dependent kinase inhibitors: brakes of the cell cycle engine during development. Bioessays. 1998. 29:1020–1029.

7. Catzavelos C, Bhattacharya N, Ung YC, Wilson JA, Roncari L, Sandhu C, et al. Decreased levels of the cell- cyclin inhibitor p27kip1 protein: prognostic implications in primary breast cancer. Nat Med. 1997. 3:227–230.

9. American Joint Committee on Cancer. AJCC Cancer Staging Manual. 2002. 6th ed. New York: Springer-Verlag;99–103.
10. Wattel E, Preudhomme C, Hecquet B, Vanrumbeke M, Quesnel B, Dervite I, et al. p53 mutations are associated with resistance to chemotherapy and short survival in hematologic malignancies. Blood. 1994. 84:3148–3157.

11. Pines J, Hunter T. Human cyclin A is adenovirus E1A- associated protein p60 and behaves differently from cyclin B. Nature. 1990. 346:760–763.

12. Murakami H, Furihata M, Ohtsuki Y, Ogoshi S. Determination of the prognostic significance of cyclin B1 overexpression in patients with esophageal squamous cell carcinoma. Virchows Arch. 1999. 434:153–158.

13. Soria JC, Jang SJ, Khuri FR, Hassan K, Liu D, Hong WK, et al. Overexpression of cyclin B1 in early-stage non-small cell lung cancer and its clinical implication. Cancer Res. 2000. 60:4000–4004.
14. Ito Y, Takeda T, Sakon M, Monden M, Tsujimoto M, Matsuura N. Expression and prognostic role of cyclin-dependent kinase 1(cdc2) in hepatocellular carcinoma. Oncology. 2000. 59:68–74.

15. Park YJ, Wen J, Bang S, Park SW, Song SY. [6]-Gingerol induces cell cycle arrest and cell death of mutant p53-expressing pancreatic cancer cells. Yonsei Med J. 2006. 47:688–697.

16. Jeong J, Park YN, Park JS, Yoon DS, Chi HS, Kim BR. Clinical significance of p16 protein expression loss and aberrant p53 protein expression in pancreatic cancer. Yonsei Med J. 2005. 46:519–525.

17. Fernández-Figueras MT, Casalots A, Puig L, Llatjós R, Ferrándiz C, Ariza A. Proliferating trichilemmal tumour: p53 immunoreactivity in association with p27Kip1 over-expression indicates a low-grade carcinoma profile. Histopathology. 2000. 38:454–457.

18. Baker SJ, Fearon ER, Nigro JM, Hamilton SR, Preisinger AC, Jessup JM, et al. Chromosome 17 deletions and p53 gene mutations in colorectal carcinomas. Science. 1989. 244:217–221.

21. Lee PL, Kim JS, Yeon HC, Lee YC, Lee SU, Kim DW, et al. Correlation between p53 and p27kip1 expression and clinicopathologic features in hepatocellular carcinoma. J Korean Cancer Assoc. 2000. 32:390–397.




 PDF
PDF ePub
ePub Citation
Citation Print
Print







 XML Download
XML Download