Abstract
Purpose
Hepatic stellate cells (HSC) are a type of pericyte with varying characteristics according to their location. However, the electrophysiological properties of HSC are not completely understood. Therefore, this study investigated the difference in the voltage-dependent K+ currents in HSC.
Materials and Methods
The voltage-dependent K+ currents in rat HSC were evaluated using the whole cell configuration of the patch-clamp technique.
Results
Four different types of voltage-dependent K+ currents in HSC were identified based on the outward and inward K+ currents. Type D had the dominant delayed rectifier K+ current, and type A had the dominant transient outward K+ current. Type I had an inwardly rectifying K+ current, whereas the non-type I did not. TEA (5mM) and 4-AP (2mM) suppressed the outward K+ currents differentially in type D and A. Changing the holding potential from -80 to -40mV reduced the amplitude of the transient outward K+ currents in type A. The inwardly rectifying K+ currents either declined markedly or were sustained in type I during the hyperpolarizing step pulses from -120 to -150mV.
Pericytes are distributed in almost all tissues and organ systems, even though their density and features in different tissues can vary. These cells are found on the microvasculature, where they encircle the outer surface and constitute a well-organized meshwork by their long processes. Therefore, they are considered to be a component of the vasculature.1,2 These cells have various functions including the regulation of capillary blood flow by altering their contractility and the regulation of new capillary growth, in addition to working as vascular smooth muscle cell precursors.1,3 They show morphological and functional differences within the same tissue or even within a single capillary bed.2 Pericytes on the larger venules tend to be longer and exhibit more contact with the endothelium.1 In addition, pericytes on the venous capillaries and post-capillary venules are more numerous and have more extensive processes than those on the arterial side of capillaries.
Hepatic stellate cells (HSC) are also known as Ito cells or fat storing cells, and are located in the space of Disse. Their long and branching cytoplasmic processes maintain contact with hepatocytes and embrace the sinusoid through the adjacent periodic side-branches.4 HSC are thought to function as liver-specific pericytes on account of their anatomical location and similarities to pericytes. Hence, HSC are believed to regulate the microcirculation of the hepatic sinusoid. This belief has been suggested in studies in which HSC were induced to contract by vasoconstrictors coupled with an increase in the intracellular Ca2+ concentration.5,6 In addition, HSC play a major role in vitamin A metabolism, extracellular matrix accumulation and the recruitment of inflammatory cells during liver repair reactions.4,7,8 Several characteristics of HSC vary according to their location within the liver lobule as different pericytes in the same tissue.9-11 Using the classic division of the liver lobule into three zones, HSC in each zone have distinct properties in terms of shape, size, vitamin A-lipid droplets, branching processes, and desmin immunoreactivity. The intralobular heterogeneity of HSC might reflect the differences in the metabolic handling of vitamin A as well as in the regulation of sinusoidal tone.4
These diverse functions and morphologies of pericytes and HSC may accompany not only different cellular components but also different electrophysiological properties. Although many studies on pericytes had attempted to demonstrate the electrophysiological characteristics, there have been few studies that have examined the electrophysiological differences.12-14 Previous studies of HSC suggested an electrophysiological profile, but they cannot explain the differences in the ionic channels of HSC.15-18 Therefore, this study examined the voltage-dependent K+ currents in cultured HSC for the investigation of heterogeneous HSC populations.
The HSC were isolated from male Sprague Dawley rats (150-250g). The rats were anesthetized intraperitoneally with ketamine at 5mg/100g. The HSC were isolated using in situ perfusion with collagenase digestion and a Nycodenz gradient centrifugation method, as described elsewhere.15,19 Briefly, the liver was perfused with Hank's buffer containing pronase (Boehringer Mannheim, Mannheim, Germany) and collagenase (Boehringer Mannheim). After the liver had been digested, it was rapidly excised and redigested with Hank's buffer containing pronase. After several washes with Hank's buffer, the cells were mixed with Hank's buffer and Nycodenz (Sigma Chemical Co, St Louis, MO, USA). After centrifugation, the HSC were aspirated, washed with DMEM (GIBCO, Grand Island, NY, USA), and cultured in 5% CO2, 95% air using DMEM containing antibiotics-antimycotics (GIBCO) and 10% fetal bovine serum (GIBCO). After plating the cells, the culture medium was changed every third day. All the experiments were performed with primary HSC that were 16 days old or less. The HSC were identified based on their typical shape and immunohistochemical staining with the antibodies against desmin and α-smooth muscle actin.20,21
The macroscopic currents were recorded using the whole-cell patch clamp technique with a patch clamp amplifier (EPC9, Instrutech Corp., Washington, NY, USA). For each experiment, glass coverslips bearing the HSC were transferred to the recording chamber mounted on the mechanical stage of an inverted microscope and superfused at 2mL/min with a normal bath solution by gravity. The patch electrodes were fabricated from a borosilicate glass capillary (Garner Glass Co., Claremont, CA, USA) using a P-97 Flaming Brown micropipette puller (Sutter Instrument Co., San Rafael, CA, USA). The patch electrodes were fire polished on a microforge (Narishige, Tokyo, Japan) and had resistances of between 1 and 3MΩ when filled with the internal solutions described below. An Ag/AgCl wire was used to ground the bath. For the voltage clamp measurements, the cell membrane capacitance and series resistance were compensated for (> 80%) electronically using an amplifier. Voltage protocol generation and data acquisition were performed using Pulse/Pulsefi (v8.50) software (Heka Elektronik, Lambrecht, Germany). The sampling rate was 1-2kHz, and the current traces were low-pass filtered using the four-pole Bessel filter in the amplifier. All the experiments were performed at room temperature (20-24℃). Drugs were applied to a single cell through a gravity-fed fused silica capillary tube connected to an array of six polyethylene tubes, located within 100µm of the cells.
When recording the K+ currents, the pipette solution contained (in mM) 5 NaCl, 135 KCl, 10 EGTA, 1 MgCl2, 10 HEPES, 0.3 MgATP, and 0.1 Na2GTP (pH 7.2 with KOH). The external solution contained (in mM) 135 NaCl, 5 KCl, 1 MgCl2, 2 CaCl2, 10 HEPES, and 10 glucose (pH 7.4 with NaOH). Tetraethylammonium chloride (TEA), BaCl2, and 4-aminopyridine (4-AP) were obtained from Sigma Chemical Co.
The amplitudes of the K+ currents were usually determined at the peak after the onset of the test pulse. The data are presented as the mean ± SEM. Statistical significance was determined using a Student's t-test. A p value < 0.05 was considered significant. The percentage of current inhibition was quantified using the following equation: [1-(Itest)/Icontrol] × 100.
The voltage-dependent K+ currents in HSC were investigated by performing patch-clamp experiments using the conventional whole-cell configuration. HSC cultured within 6 days after isolation were used in these experiments. The current-voltage (I-V) relationship obtained during the voltage ramp pulses from -140 to 60mV showed an outward K+ current that was activated at the depolarized potentials and an inward K+ current that was activated at hyperpolarized potentials (Fig. 1). The HSC could be classified into four types according to the component of the K+ current (Fig. 1). Taking into consideration the activation of the outward K+ current, the HSC were divided into two different subpopulations, one consisting of mainly delayed rectifier K+ currents (type D) and the other comprising mainly transient outward K+ currents (type A). Type I showed an inwardly rectifying K+ current between -140 and -80mV while non-type I did not. Type ID and IA were type D and A with the inwardly rectifying K+ current, respectively. The proportion of each type in the HSC was uneven. Of the 79 cells tested, 34 were type D (43.1%), 20 were type ID (25.3%), 17 were type IA (21.5%), and 8 were type A (10.1%).
The voltage-dependent outward K+ currents were further investigated to determine the difference between type D and A. Fig. 2 shows the outward K+ currents evoked by a series of depolarizing step pulses ranging from -80 to 70mV from a holding potential of -80mV. At voltages more positive than -40mV, outward K+ currents were produced. As shown in Fig. 2A, the outward K+ currents were not inactivated for a 200-ms step in type D. However, the currents were inactivated in type A, showing significant time-dependent inactivation. The I-V relationships showed linear outward rectification in type D and curved outward rectification in type A (Fig. 2B). The outward currents of type A were activated at a less depolarized pulse and were significantly larger at a voltage ranging from -30mV to 40mV than those of type D. An average current amplitude of type D at 20mV was 19.0 ± 2.7pA/pF (n = 48) and that of type A was 18.7 ± 2.8pA/pF (n = 22). Type D and type A showed a mean capacitance of 17.7 ± 4.3pF (n = 53) and 26.2 ± 3.7pF (n = 25), respectively.
The effect of TEA and 4-AP was tested to determine the different constituents of the voltage-dependent outward K+ currents. TEA and 4-AP were used to block the outward K+ currents at several concentrations (100µM-10mM) and had their maximum effect at 5mM and 2mM, respectively. Adding 5mM TEA to the extracellular solution led to a similar decrease in both the type K+ currents elicited by a step pulse to 20 mV (Fig. 3 upper row). Extracellular TEA reduced the outward K+ current in type D (n = 15) and A (n = 12) by 28.2 ± 2.7% and 35.1 ± 3.6%, respectively. In the K+ currents elicited by the voltage ramp pulse, the portion inhibited by TEA in the outward K+ currents was larger as the cells became more depolarized in both types (Fig. 3 lower row). Fig. 4 shows the inhibition of the outward K+ current by 2mM 4-AP. At 20mV, extracellular 4-AP reduced the outward K+ current in type D (n = 8) and A (n = 8) by 54.1 ± 3.2% and 75.4 ± 3.7%, respectively (Fig. 4 upper row). The inhibition by 4-AP was larger than that by TEA (p < 0.001). The outward K+ currents were decreased more in the peak than in the sustained portion, and the remaining currents did not decline in type A. In the K+ currents elicited by ramp pulse, the transient outward K+ current in type A was mostly inhibited (Fig. 4 lower row). After the application of both TEA and 4-AP, the outward K+ currents in type D (n = 5) and A (n = 5) were blocked by 79.6 ± 3.8% and 91.9 ± 2.1%, respectively. This suggests that the voltage-dependent outward K+ currents are mainly mediated by the opening of TEA-sensitive and 4-AP-sensitive K+ channels and that both types have a different composition of K+ channels.
The transient outward (A-type) K+ current has the half maximal inactivation value of -75mV.16,22 When the holding potential was changed from -80mV to -40mV, the currents evoked by serial pulses were largely decreased in both type A and D and the inactivation over a 200 ms pulse disappeared in type A (Fig. 5). This procedure decreased the outward K+ currents in type D (n = 5) and type A at 20mV (n = 5) by 41.2 ± 2.8% and 71.5 ± 3.3%, respectively. The steady-state inactivation of type A was larger than that of type D (p < 0.0001). Therefore, the transient outward K+ currents in type A were almost completely abolished. These results suggest that the transient outward K+ current is dominant in type A and the delayed rectifier K+ current is dominant in type D, which causes the distinguishing features of the outward K+ current.
The inward K+ current at the hyperpolarized potentials was examined in more detail. Hyperpolarizing pulses elicited inward K+ currents, and the amplitude of these currents increased with further hyperpolarization in some of the tested HSC (Fig. Fig. 6A). The I-V relationships showed inward rectification within a command voltage less than -70mV (Fig. 6B). In order to differentiate a subpopulation with an inwardly rectifying K+ current from the others lacking it, the former was defined as type I occupying 46.8% of the tested HSC. The average current amplitude of type I was -16.9 ± 2.8 pA/pF at -100mV from a holding potential of -50mV (n = 15). The HSC without the inwardly rectifying K+ currents (non-type I) had a mean capacitance of 13.4 ± 3.5pF (n = 36). In contrast, the capacitance of type I was 28.7 ± 4.9pF (n = 29, p < 0.05). The application of Ba2+ (200µM) to the extracellular solution led to the almost complete and reversible inhibition of the inwardly rectifying K+ current (data not shown). Some of the inwardly rectifying K+ currents showed a significant time-dependent decrease at the very negative hyperpolarizing pulses (Fig. 6C). The I-V relationship of the peak inward K+ current was almost linear at potentials more negative than -70 mV (Fig. 6D). The steady-state current reached a maximum at -120mV, and the I-V relation showed a region of negative slope at more negative potentials. However, the other inwardly rectifying K+ currents decayed slightly or were sustained for 1-s pulses (Fig. 6C). The I-V relationships of the peak and steady-state currents were linear and positive slopes (Fig. 6D). This suggests that the inwardly rectifying K+ currents show distinct time dependent changes, such as decline and sustaining, which provide clues to the various HSC populations with different physiological properties.
As the duration of the culture was increased, the HSC were transformed to the activated phenotype. The ratio of the four types as the HSC culture aged was analyzed to determine the relationship between the K+ currents and activation. The proportion of type A (including type IA) was 12.5% at 1-3 days (n = 24). There was a transient increase to 69.2% at 8-10 days (n = 13) and then a decrease to 27.2% at 14-16 days (n = 11). The proportion of type I was 62.5% at 1-3 days (n = 24). This proportion decreased to 47.1% at 4-7 days (n = 17), and then continually to 27.3% at 14-16 days (n = 11). In addition, the mean capacitance of the HSC increased along with the culture's age. HSC cultured for 1-3 days had a capacitance of 3.6 ± 0.4pF (n = 29), and those cultured for 14-16 days had 49.8 ± 6.2pF (n = 14, p < 0.01).
This study examined the voltage-dependent K+ currents responsible for determining the membrane potential. This paper reports the electrophysiological characterization of the voltage-dependent K+ currents of cultured HSC and the differences in their components. The results suggest four different types of K+ currents: D, A, ID, and IA. These types were set according to the property of an outward K+ current and the presence of an inwardly rectifying K+ current. To our knowledge, this study is unique in that it identified the various HSC with different components of the voltage-dependent K+ currents.
Pericytes in different organs or tissues are heterogeneous in structure, morphology, and function. Even pericytes on the venous sides of capillary beds are different from those on the arterial sides and those within the same tissue or even within a single capillary bed are different.1,2 HSC show various properties like pericytes according to their location within the liver lobule.9-11 HSC located in the periportal vein area and in the pericentral vein area contain minute vitamin A-lipid droplets. Both have short branching process and are heterogeneous in their appearance, size, and desmin immunoreactivity. HSC located in the lobular area store abundant vitamin A-lipid droplets and extend encompassing processes. In addition, the electrophysiological properties in HSC, including the K+ currents, may be different at various locations in the liver. The K+ currents found in the smooth muscles and pericytes had somewhat different components according to the tissues and cells.12-14,23 An inwardly rectifying K+ current was found in the smooth muscle cells of the cerebral and coronary arteries but not in other tissues.22,24,25 In coronary pericytes and smooth muscle cells from the small bronchioles, there are discrepancies in expressing the inwardly rectifying K+ current and the properties of the outward K+ current.14,26
Kashiwagi et al. suggested that the transient outward K+ current in HSC were the major component of the outward K+ current.16 They demonstrated that the transient outward K+ currents decayed during the depolarizing pulse and showed two (steep- and gentle-) slant rectification in I-V relationships. In this study, the outward K+ currents had different I-V relationships and time- dependent inactivations. In the ramp pulse, the slope of the outward K+ current was linear in type D but curved in type A. In addition, the current evoked by the step pulses decayed significantly in type A. The amplitudes of the currents in type A were greater than those in type D in the voltage range from -30mV to 40mV. Both types were similarly sensitive to TEA but were differentially sensitive to 4-AP. Type A was inhibited more by 4-AP than type D, with its inactivation diminished during the depolarizing pulse. By changing the holding potential from -80 mV to -40mV in type A, the decline during the pulse disappeared. The diminution in type A was significantly larger than in type D. Overall, it is believed that the major component of the voltage- dependent outward K+ currents in type D was the delayed rectifier K+ current while that in type A was the transient outward K+ current.
The voltage-dependent outward K+ channels in the HSC have also been identified in other cell types. In action potential-generating cells such as neurons and cardiac muscle cells, this channel plays an important role in the repolarization of the action potential as well as in the regulation of the membrane potential.22,23 In vascular smooth muscle cells, which do not generate action potentials, the outward K+ channel acts mainly to limit the extent of membrane depolarization.27 This is because the voltage-dependent K+ channels open when the membrane potential is depolarized and K+ efflux through K+ channels increases with depolarization through voltage-dependent activation.22 Although the actual physiological role of the voltage-dependent outward K+ channels in HSC requires further examination, it is believed that the outward K+ channels may be a major factor in the regulation of the membrane potential. In this view, type A may have greater ability to stabilize membrane depolarization than type D has. The voltage-dependent and subsequent events, such as the activation of the voltage-gated channels, increase in the intracellular Ca2+, and the Ca2+ dependent machinery may be less activated in type A than in type D.
The inward K+ current of the HSC shared the distinct properties of currents mediated by the classical inward rectifier K+ channels. The inward rectifier K+ channels are present in a variety of excitatory and non-excitatory cells. The physiological roles of the inward rectifier K+ channels in these cells include regulating the resting membrane potential, preventing membrane hyperpolarization to values more negative than the equilibrium potential of K+, and minimizing the loss of cellular K+.22 Elsewhere in the arterial smooth muscle, the inward rectifier K+ channel regulates the membrane potential and mediates the K+-induced dilations.23 Therefore, the inward rectifier K+ channel in type I HSC serves as a device responsible for maintaining the membrane potential, and the non-type I HSC may be less able to compensate for hyperpolarizing stress.
There were two types of responses to the very negative hyperpolarization of the inward rectifier K+ channels in the time dependent inactivation, declining and sustaining. Consistent with findings in cardiac myocytes and coronary arterial smooth muscle cells,28,29 the inwardly rectifying K+ currents declined during the very negative hyperpolarizing steps from -120 to -150mV. Some studies dealing with native cells expressing the endogenous inward rectifier K+ channels reported that the inactivation of the inwardly rectifying K+ currents made a "crisscrossing pattern".26,28,29 Although the reason for the different inactivation patterns was not determined, it was examined that external Na+ blocked the highly voltage-dependent inactivation of the inward rectifier K+ channels.28,29
HSC proliferate around injured sites and transform from the quiescent phenotype to the activated phenotype. The process of activation is characterized by various morphological and functional changes. The activation may alter the expression of the ionic channels contributing to the contractility, and it was found that the expression of the L-type Ca2+ channel increased during activation.6,18 These results highlight the decrease in type I population and the transient increase in type A population as the culture aged for approximately 2 weeks. This indicates that the inwardly rectifying K+ current decreases and the transient outward K+ current increases then decreases thereafter. The L-type Ca2+ channel is activated at a membrane potential > -10mV, reached a maximum current at almost 0-10mV, and was inactivated at -60 to -10mV.22 The activation of the Ca2+ channel evokes the influx of Ca2+ into HSC. This causes an increase in intracellular Ca2+ concentration, which induces significant changes in the intracellular environment and enforces the numerous Ca2+ dependent signaling. This phenomenon might be an inevitable stress to activated HSC and could be relieved by developing a protective or handling mechanism for the influx of Ca2+. Therefore, activation is necessary to induce a series of protective changes either before the expression of the Ca2+ channel or at the same time. It is possible that the transient outward K+ current is one of the protective mechanisms because it is activated at more than half maximum to stabilize the depolarization of the membrane potential at almost -20-10mV, which is the voltage range of the threshold and the maximal activation of the L-type Ca2+ channel. Hence, the proportion of type A increased transiently at 8-10 days when the Ca2+ channel was expressed and then declined at 14-16 days when the other Ca2+ handling machineries might have developed. In addition, a decrease in the inwardly rectifying K+ current might depolarize the resting membrane potential, which would cause the steady-state inactivation of the Ca2+ channel. Therefore, it also plays a role as a protective mechanism against the enhanced influx of Ca2+. Further study with various approaches will be needed to determine if the transient outward K+ current and inwardly rectifying K+ current have protective roles against the sudden influx of Ca2+.
These results suggest that there are four types of voltage-dependent K+ currents expressed in HSC. Each has a voltage-dependent outward K+ current, transient outward or delayed rectifier, and half of the HSC have inwardly rectifying K+ currents. These results show the different HSC populations in the hepatic sinusoidal system, along with the properties of the various voltage-dependent K+ channels expressed in HSC.
Figures and Tables
 | Fig. 1Four types of voltage-dependent K+ currents in the cultured HSC. Voltage-dependent alterations in the cultured HSC membrane currents were measured under voltage-clamp conditions using the whole cell configuration of the patch-clamp technique. Representative traces of K+ currents in HSC cultured for 2 days were illustrated as type D (dominant delayed rectifier outward K+ current), type A (dominant transient outward K+ current), type ID (type D with inwardly rectifying K+ current), and type IA (type A with inwardly rectifying K+ current). K+ currents were elicited by the voltage ramps from -140 to 60mV in cells held at -80mV. |
 | Fig. 2Two types of voltage-dependent outward K+ currents. (A) Representative traces show type D and type A outward K+ currents in HSC cultured for 3 days. The currents were evoked by a series of depolarizing step pulses ranging from -80 to 70mV in 10mV increments for 200 ms, from a holding potential of -80mV. (B) Relationship between the command voltages and the outward K+ currents (n = 13 for type D, n = 7 for type A). The data are shown in mean ± SEM. |
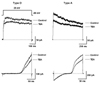 | Fig. 3Inhibitory effect of TEA on the voltage-dependent outward K+ currents. Representative traces of type D and A outward K+ currents were measured before and after the application of TEA (5mM) in HSC cultured for 2 days. The outward K+ currents were elicited by the step pulse to 20mV (Upper) and by the ramp pulse from -140 to 60 mV in cells held at -80mV (Lower). |
 | Fig. 4Inhibitory effect of 4-AP on the voltage-dependent outward K+ currents. Representative traces of type D and A outward K+ currents were measured before and after the application of 4-AP (2mM) in HSC cultured for 2 days. The outward K+ currents were elicited by the step pulse (Upper) and by the ramp pulse (Lower). |
 | Fig. 5Effect of holding potential on the voltage-dependent outward K+ currents. (A) Current responses in type D and A HSC cultured for 3 days were evoked by pulses from a different holding potential (Vh) of -80mV and -40mV with the voltage-clamp protocol shown in Fig. 2. (B) The peak amplitudes of the currents were plotted as a function of the voltages at different holding potentials (-80mV and -40mV). |
 | Fig. 6Inwardly rectifying K+ currents and time-dependent inactivation in cultured HSC. (A) Representative traces show inwardly rectifying K+ currents (type I) and inward K+ current which are not inwardly rectifying (non-type I) in HSC cultured for 5 days. The inward K+ currents were evoked by a series of voltage pulses ranging from -110 to -30mV with 10mV increments from a holding potential of -50mV. (B) Relationships between the command voltages and the inward K+ currents at the peak (n = 5). (C) At the test potentials ranging from -150 to -120mV, the inwardly rectifying K+ current showed significant time-dependent inactivation (decline type I) and sustained activation (sustaining type I) in HSC cultured for 5 days. (D) The peak and steady- state currents were plotted as a function of the voltages (n = 5). The data are shown in mean ± SEM. |
References
2. Rucker HK, Wynder HJ, Thomas WE. Cellular mechanisms of CNS pericytes. Brain Res Bull. 2000. 51:363–369.

4. Pinzani M. Novel insights into the biology and physiology of the Ito cell. Pharmacol Ther. 1995. 66:387–412.

5. Kawada N, Tran-Thi TA, Klein H, Decker K. The contraction of hepatic stellate (Ito) cells stimulated with vasoactive substances. Possible involvement of endothelin 1 and nitric oxide in the regulation of the sinusoidal tonus. Eur J Biochem. 1993. 213:815–823.

6. Pinzani M, Failli P, Ruocco C, Casini A, Milani S, Baldi E, et al. Fat-storing cells as liver-specific pericytes. Spatial dynamics of agonist-stimulated intracellular calcium transients. J Clin Invest. 1992. 90:642–646.

7. Hautekeete ML, Geerts A. The hepatic stellate (Ito) cell: its role in human liver disease. Virchows Arch. 1997. 430:195–207.

8. Knittel T, Dinter C, Kobold D, Neubauer K, Mehde M, Eichhorst S, et al. Expression and regulation of cell adhesion molecules by hepatic stellate cells (HSC) of rat liver: involvement of HSC in recruitment of inflammatory cells during hepatic tissue repair. Am J Pathol. 1999. 154:153–167.

9. Ballardini G, Fallani M, Biagini G, Bianchi FB, Pisi E. Desmin and actin in the identification of Ito cells and in monitoring their evolution to myofibroblasts in experimental liver fibrosis. Virchows Arch B Cell Pathol Incl Mol Pathol. 1988. 56:45–49.

10. Ballardini G, Groff P, Badiali de Giorgi L, Schuppan D, Bianchi FB. Ito cell heterogeneity: desmin-negative Ito cells in normal rat liver. Hepatology. 1994. 19:440–446.

11. Wake K, Sato T. Intralobular heterogeneity of perisinusoidal stellate cells in porcine liver. Cell Tissue Res. 1993. 273:227–237.

12. Zhang Z, Rhinehart K, Pallone TL. Membrane potential controls calcium entry into descending vasa recta pericytes. Am J Physiol Regul Integr Comp Physiol. 2002. 283:R949–R957.
13. Sakagami K, Wu DM, Puro DG. Physiology of rat retinal pericytes: modulation of ion channel activity by serum-derived molecules. J Physiol. 1999. 521:637–650.

14. von Beckerath N, Nees S, Neumann FJ, Krebs B, Juchem G, Schömig A. An inward rectifier and a voltage-dependent K+ current in single, cultured pericytes from bovine heart. Cardiovasc Res. 2000. 46:569–578.

15. Lee KI, Kong ID, Baik SK, Kim HS, Lee DK, Kwon SO, et al. Characteristics of potassium and calcium currents of hepatic stellate cells (ito) in rat. Yonsei Med J. 2004. 45:649–660.

16. Kashiwagi S, Suematsu M, Wakabayashi Y, Kawada N, Tachibana M, Koizumi A, et al. Electrophysiological characterization of cultured hepatic stellate cells in rats. Am J Physiol. 1997. 272:G742–G750.

17. Gasull X, Batallr R, Ginès P, Sancho-Bru P, Nicolás JM, Görbig MN, et al. Human myofibroblastic hepatic stellate cells express Ca2+-activated K+ channels that modulate the effects of endothelin-1 and nitric oxide. J Hepatol. 2001. 35:739–748.

18. Bataller R, Gasull X, Ginès P, Hellemans K, Görbig MN, Nicols JM, et al. In vitro and in vivo activation of rat hepatic stellate cells results in de novo expression of L-type voltage-operated calcium channels. Hepatology. 2001. 33:956–962.

19. Bedossa P, Houglum K, Trautwein C, Holstege A, Chojkier M. Stimulation of collagen alpha 1(I) gene expression is associated with lipid peroxidation in hepatocellular injury: a link to tissue fibrosis? Hepatology. 1994. 19:1262–1271.

20. Yokoi Y, Namihisa T, Kuroda H, Komatsu I, Miyazaki A, Watanabe S, et al. Immunocytochemical detection of desmin in fat-storing cells (Ito cells). Hepatology. 1984. 4:709–714.

21. Rockey DC, Boyles JK, Gabbiani G, Friedman SL. Rat hepatic lipocytes express smooth muscle actin upon activation in vivo and in culture. J Submicrosc Cytol Pathol. 1992. 24:193–203.
22. Hille B. Ionic Channels of Excitable Membranes. 1992. Sunderland, MA: Sinauer.
23. Nelson MT, Quayle JM. Physiological roles and properties of potassium channels in arterial smooth muscle. Am J Physiol. 1995. 268:C799–C822.

24. Edwards FR, Hirst GD, Silverberg GD. Inward rectification in rat cerebral arterioles; involvement of potassium ions in autoregulation. J Physiol. 1988. 404:455–466.

25. Robertson BE, Bonev AD, Nelson MT. Inward rectifier K+ currents in smooth muscle cells from rat coronary arteries: block by Mg2+, Ca2+, and Ba2+. Am J Physiol. 1996. 271:H696–H705.
26. Snetkov VA, Ward JP. Ion currents in smooth muscle cells from human small bronchioles: presence of an inward rectifier K+ current and three types of large conductance K+ channel. Exp Physiol. 1999. 84:835–846.

27. Hirst GD, Edwards FR. Sympathetic neuroeffector transmission in arteries and arterioles. Physiol Rev. 1989. 69:546–604.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download