Abstract
Purpose
Bisphosphonates have been used to treat osteoporosis for more than ten years. However, complications associated with long-term administration of bisphosphonates, such as nonunion after pelvic insufficiency fracture or osteonecrosis of the jaw, have been recently reported in the literature. We investigated the relationships among the mechanical properties of the intact rat femur as well as healing fracture calluses and the intraosseous concentration of pamidronate (ICP), after long-term administration of pamidronate in a rat osteoporosis model.
Materials and Methods
We performed bilateral ovariectomy in 25 3-month-old female Sprague-Dawley rats. Beginning three months after ovariectomy, disodium pamidronate (0.5mg/kg) was injected every month. After the six-month administration period, the left femoral shaft was fractured using the closed fracture technique. Five weeks after fracture, 23 rats were euthanized and both femora were removed. We checked the mechanical properties of the intact (right) and fractured (left) femora using a three-point bending technique. Intraosseous concentration of pamidronate was checked by high-performance liquid chromatography.
Results
The mean ICP was 61.8 ± 15.7ng/mg of bone. High ICP decreased the ultimate load to failure, stiffness, and ultimate stress of the intact femora (p = 0.015, 0.027, 0.039, respectively). There was a tendency to decrease the ultimate load to failure in the healing callus when the ICP increased (p = 0.183). High ICP decreased the bone mineral density of the femoral head (p = 0.005).
Bisphosphonates constitute one of the most popular classes of drugs used in the treatment of osteoporosis. Clinical results after ten-year's administration of alendronate are promising.1,2 However, complications associated with long-term administration of bisphosphonates, such as nonunion after pelvic insufficiency fracture3,4 or osteonecrosis of the jaw,5,6 have been reported recently in the literature. The mechanisms behind these events are not yet clear. The most plausible cause is their strong antiresorptive effect on bone. Since they suppress osteoclast-mediated normal and abnormal bone remodeling, the weakening of bone due to accumulated microstructural damage and deficient repair processes is suspected.7 Bisphosphonates have a strong affinity for hydroxyapatite, and their skeletal half-lives seem to be appreciable after they have been incorporated into bone. Therefore, it is important to determine whether the intraosseous accumulation of these agents compromises bone strength and the fracture healing process. We investigated the relationships among the mechanical properties of intact rat femora as well as healing fracture calluses and pamidronate concentrations in bone after long-term administration of pamidronate in a rat osteoporosis model. Pamidronate is an amino-group-containing bisphosphonate, with a half-life of approximately 90-140 days.8
Twenty-five 3-month-old female Sprague-Dawley rats (body weight, 350 ± 30gm) were used in this study. During the experimental period, three to four rats were housed in one cage and allowed free access to water and pelleted commercial rodent diet. Bilateral ovariectomy was performed through the dorsal approach under general anesthesia by the intraperitoneal (i.p.) injection of ketamine sulfate (60mg/kg) and xylazine sodium (10mg/kg). Three months after ovariectomy, disodium pamidronate (0.5mg/kg) was injected i.p. every month for 6 months. After the six-month administration period, the left femoral shaft was fractured using the closed fracture technique advocated by Bonnarens and Einhorn.9 The fracture was made at the mid-shaft of the femur, and anteroposterior and lateral x-rays were taken to confirm a linear fracture at the mid-diaphysis. Two rats were excluded from the study because of comminution at the fracture site, which showed gross instability. Pamidronate administration was discontinued from this point. Five weeks after fracture, the rats were euthanized with ether. Both femora were removed and all soft tissues, except the periosteum or soft callus, were stripped. Both femora were wrapped with a saline-soaked gauze and stored at -20℃ until further study.
Frozen femora were thawed at room temperature for 2 hours before testing. Mechanical tests were performed on intact (right) and fractured (left) femora using a destructive three-point bending procedure. Initially, an intramedullary Kirschner wire was removed through the intercondylar notch of the left distal femur. The femur was placed supine on two round bars at a distance of 15mm in a mechanical testing machine (Instron model 6022, Instron Co., Norwood, MA, USA) and deflected by a notched bar on the opposite side of the bone. The descending speed of the notched bar was 1mm per minute. Load and deflection were recorded. Outer and inner diameters of the femoral shaft were measured with a micro-caliper after mechanical testing. The ultimate load to failure, stiffness, and ultimate stress were calculated from the load deflection curves.10
The bone mineral density of the right femoral head was measured by dual energy x-ray absorptiometry (Hologic QDR 4500A, Bedford, OH, USA) after biomechanical testing. The right proximal femur was placed on the examination table in a supine position, and the scan was performed in Hi-resolution mode, suitable for small animals (version 11.2:3). A region of interest was manually drawn along the edge of the femoral head. The right femur was frozen to -20℃ immediately after testing for pamidronate concentration in the bone.
Pamidronate disodium, and the internal standard (IS, pentamethylene-1,1-bisphosphonate monosodium salt, BFI 8739, Gador S.A., Buenos Aires, Argentina) were dissolved in water to a concentration of 50µg/mL and 20µg/mL, respectively. These solutions were stored at 4℃ and used as stock solutions.
The HPLC system consisted of a PU-980 (JASCO, Tokyo, Japan) series pump, an AS-950 (JASCO, Tokyo, Japan) autosampler, a FP-920 (JASCO, Tokyo, Japan) fluorescence detector, and a PLRP-S column (15 × 0.46cm I.D., Phenomenex, Torrance, CA, USA). Data acquisition and analysis were performed using Millennium 32 software (Waters, Milford, MA, USA). The mobile phase consisted of 16% acetonitrile in 0.025M citrate-phosphate buffer, pH 6.5, delivered at a flow-rate of 1.0mL/min. The eluate was monitored fluorometrically at excitation and emission wavelengths of 436 and 508nm, respectively.
Twelve and a half milligrams of finely ground and sifted (< 250µm) powder of the diaphysis were dissolved in 1mL of 0.2M HCl; a 50µL aliquot of IS solution was added to each sample. Spiked bone controls consisted of bone powder without endogenous pamidronate disodium that had been spiked with various amounts of pamidronate disodium. Samples were vortexed and incubated overnight at room temperature. After centrifugation, 250µL aliquots, equivalent to 3.13mg of bone were diluted with 0.5mL of 0.01M NaOH and 25µL of 10M NaOH to co-precipitate the pamidronate disodium with calcium phosphate. The precipitate was isolated by centrifugation (1000g, 10 min) and the pellets obtained were washed with 0.5mL of water to remove any trapped amine-containing compounds. After further centrifugation, the supernatant was discarded and the calcium salt pellet was dissolved in 300µL of 0.2M phosphoric acid. To remove calcium from the dissolved sample, 375µL of 0.2M EDTA in 0.2M NaOH, pH 10.3, was added to the sample followed by 300µL of AG 50W-X8 (K+/-form) (Bio-Rad Laboratories, Hercules, Canada). After vortexing and centrifugation, a 550µL aliquot was filtered through a 0.45µm membrane and made alkaline with 8µL of 10M NaOH. The pamidronate disodium from a 120µL aliquot of each prepared sample was derivatized by adding 120µL of 1M carbonate buffer, pH 10.7, followed by 24µL of 1mg/mL 2,3-naphthalene dicarboxaldehyde (Aldrich, Milwaukee, WI, USA) and 2µL of 1mg/mL N-acetyl-D-penicillamine (Fluka Biochemika, NY, USA). The sample was then mixed, incubated for 2 min, and a 100µL aliquot was applied to the HPLC column. Each sample was derivatized at precisely the same time before analysis. Calibration curves were constructed by plotting the peak area versus the concentration of the standard solutions injected onto the HPLC column. Unknown sample concentrations were calculated by the Millennium 32 software using the calibration curve.
Data are expressed as the mean and one standard deviation. Simple linear regression analysis was performed to test the relationship between intraosseous concentration of pamidronate (ICP) and each mechanical property of the intact femora, healing calluses and BMD of the femoral heads. A p value < 0.05 was considered significant. Statistical analysis was performed using SAS software package 8.02 version (SAS Institute, Inc., Cary, NC, USA).
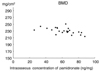
The mean ICP was 61.8 ± 15.7ng/mg. A high ICP decreased the ultimate load to failure, stiffness, and ultimate stress of the intact femora; ultimate load to failure [156.8 ± 16.5N, ultimate load to failure (N) = 189.3-0.53 × (ICP), p = 0.015, Fig. 1], stiffness [454.3 ± 120.5N/mm, stiffness (N/mm) = 672.6-3.54 × (ICP), p = 0.027, Fig. 2], ultimate stress [162.5 ± 51.1N/mm2, ultimate stress (N/mm2) = 249.8-1.41 × (ICP), p = 0.039, Fig. 3]. There was a tendency toward decreased ultimate load to failure in the healing callus when the ICP increased. It was not statistically significant [35.6 ± 12.7N, ultimate load to failure of the healing callus (N) = 50.0-0.23 × (ICP), p = 0.183, Fig. 4]. High ICP decreased the bone mineral density of the femoral head [230 ± 9mg/cm2, bone mineral density (mg/cm2) = 250-0.33 × (ICP), p = 0.005, Fig. 5].
The report of ten-year's experience with alendronate for osteoporosis in postmenopausal women revealed promising effects of bisphosphonate in increasing bone mineral density by 13.7 percent at the lumbar spine and 10.3 percent at the trochanter. Safety data did not suggest that prolonged treatment resulted in any loss of the benefit proven in short-term administration.1 However, accumulation of microstructural damage, which is normally remodeled out by osteoclasts, has been reported after long-term antiresorptive drug administration,7 and pamidronate-induced osteopetrosis has been reported in children.12 Odvina et al. reported that severe suppression of bone turnover resulted in insufficiency fractures of the pelvis, and the fractures failed to unite after long-term treatment of bisphosphonate. Microscopic findings of the bone biopsy specimens were striking. There was no visible osteoclast, osteoblast, or osteoid indicating characteristics of frozen bone.3 Moreover, the Food and Drug Administration (FDA) warns that use of bisphosphonates increases the risk of osteonecrosis of the jaw (ONJ) in multiple myeloma, metastatic bone tumor, and osteoporosis. The incidence of ONJ following the treatment of osteoporosis with bisphosphonate is not high; it occurs in approximately 0.6 patients per 10,000 patient-year. However, the deleterious effects of an exposed jaw bone (either external or internal) are tremendous.13
The main purpose of the present study was to investigate the relationships between the mechanical properties of the intact rat femur as well as the healing fracture callus and ICP after long-term administration of pamidronate in a rat osteoporosis model. The six-month administration period represented one-sixth of the life span of the rat.8 The dosage in the present study was 3 times higher than that recommended for osteoporosis in clinics.2 Administration of a high dosage of bisphosphonates may result in mineralization defects during the treatment of Paget's disease14 and fibrous dysplasia.15 High ICP slightly decreased the bone mineral density of the femoral head. In the pilot study, however, we fond that the pamidronate-treated group showed higher bone mineral density compared with the untreated group in the same osteoporotic rat model. Further histomorphometric study is necessary to scrutinize mineralization deficiencies as well as the accumulation of microcracks in this high ICP group. High ICP may lead to longer half-life, prolonged effect of the drug, impaired turnover, and accumulation of microcracks due to suppressed osteoclast-mediated bone resorption. In the present study, the right femur should have borne more weight because the left femur was fractured. This might enhance the formation and propagation of microcracks due to overload in the intact side.
Increased bone mineral density and reduced fracture incidences have been achieved by using bisphosphonates in osteoporosis. However, bisphonates cannot completely eliminate fracture episodes. The fracture healing process includes various stages such as endochondral ossification, woven bone production, and callus remodeling to lamellar bone, and the fracture callus is heterogenous with respect to the tissue composition, especially in the early stage. The reports on short-term administration of various bisphosphonates revealed no serious effects on the fracture healing process, even though they delay remodeling of the callus.16,17 However, the reports on the long-term effect of bisphosphonate on fracture healing differ greatly in study design, administration pattern, study drugs, and results.18-20 Li et al. reported that long-term administration of incadronate disodium (continuous treatment before and after fracture surgery until death) delayed the fracture healing process in growing rats, especially under high doses.19 Koivukangas et al. reported that long-term administration of clodronate (before the fracture) did not inhibit fracture healing.18 Increased incidence of nonunion after osteotomy in osteogenesis imperfecta treated with pamidronate implies that the accumulation of pamidronate in bone may cause deleterious effects on the healing process.21,22 In this study, we could not find any relationships between the mechanical properties of the healing callus and the intraosseous concentration of pamidronate. The early fracture healing process starts with recruitment of mesenchymal cells and formation of a soft callus. The soft callus is replaced by hard callus through endochondral ossification in which the osteoclast is involved to resorb the calcified chondroid tissue. We suspect that high ICP does not affect the formation of the soft callus unless pamidronate is administered continuously after fracture.
High concentrations of pamidronate in intact bone decreased the bone mineral density and weakened the mechanical strength of the rat femur. The mechanical strength of the early healing callus was not correlated with the concentration of pamidronate in bone.
Figures and Tables
 | Fig. 1High intraosseous concentration of pamidronate decreases the ultimate load to failure of the intact femur. Ultimate load to failure (N) = 189.3-0.53 × (ICP), p = 0.015. |
 | Fig. 2High intraosseous concentration of pamidronate decreases the stiffness of the intact femur. Stiffness (N/mm) = 672.6-3.54 × (ICP), p = 0.027. |
 | Fig. 3High intraosseous concentration of pamidronate decreases the ultimate stress of the intact femur. Ultimate stress (N/mm2) = 249.8-1.41 × (ICP), p = 0.039. |
References
1. Bone HG, Hosking D, Devogelaer JP, Tucci JR, Emkey RD, Tonino RP, et al. Ten year's experience with alendronate for osteoporosis in postmenopausal women. N Engl J Med. 2004. 350:1189–1199.

2. Fleisch H. Bisphosphonates in bone disease. 2000. 4th ed. San Diego: Academic Press.
3. Odvina CV, Zerwekh JE, Rao DS, Maalouf N, Gottschalk FA, Pak CY. Severely suppressed bone turnover: a potential complication of alendronate therapy. J Clin Endocrinol Metab. 2005. 90:1294–1301.

5. Maerevoet M, Martin C, Duck L. Osteonecrosis of the jaw and bisphosphonates. N Engl J Med. 2005. 353:99–102.

6. Marx RE, Sawatari Y, Fortin M, Broumand V. Bisphosphonate-induced exposed bone (osteonecrosis/osteopetrosis) of the jaws: risk factors, recognition, prevention, and treatment. J Oral Maxillofac Surg. 2005. 63:1567–1575.

7. Komatsubara S, Mori S, Mashiba T, Ito M, Li J, Kaji Y, et al. Long-term treatment of incadronate disodium accumulates microdamage but improves the trabecular bone microarchitecture in dog vertebra. J Bone Miner Res. 2003. 18:512–520.

8. Whyte MP, Wenkert D, Clements KL, McAlister WH, Mumm S. Bisphosphonate-induced osteopetrosis. N Engl J Med. 2003. 349:457–463.

9. Bonnarens F, Einhorn TA. Production of a standard closed fracture in laboratory animal bone. J Orthop Res. 1984. 2:97–101.

10. Hoggarth CR, Bennett R, Daley-Yates PT. The pharmacokinetics and distribution of pamidronate for a range of doses in the mouse. Calcif Tissue Int. 1991. 49:416–420.

11. Turner CH, Burr DB. Basic biomechanical measurements of bone: a tutorial. Bone. 1993. 14:595–608.

12. King LE, Vieth R. Extraction and measurement of pamidronate from bone samples using automated pre-column derivatization, high-performance liquid chromatography and fluorescence detection. J Chromatogr B Biomed Appl. 1996. 678:325–330.

13. Cao Y, Mori S, Mashiba T, Westmore MS, Ma L, Sato M, et al. Raloxifene, estrogen, and alendronate affect the processes of fracture repair differently in ovariectomized rats. J Bone Miner Res. 2002. 17:2237–2246.

14. Goodship AE, Walker PC, Mc Nally D, Chambers T, Green JR. Use of a bisphosphonate (pamidronate) to modulate fracture repair in ovine bone. Ann Oncol. 1994. 5:Suppl 7. S53–S55.
15. Koivukangas A, Tuukkanen J, Kippo K, Jämsä T, Hannuniemi R, Pasanen I, et al. Long-term administration of clodronate does not prevent fracture healing in rats. Clin Orthop Relat Res. 2003. 408:268–278.

16. Li J, Mori S, Kaji Y, Mashiba T, Kawanishi J, Norimatsu H. Effect of bisphosphonate (incadronate) on fracture healing of long bones in rats. J Bone Miner Res. 1999. 14:969–979.

17. Yang KH, Park SY, Yoo JH, Kim TH, Park HW, Ryu JH, et al. Effect of the long-term administration of pamidronate on bone strength and fracture healing in a rat model. J Korean Orthop Assoc. 2005. 40:1043–1049.
18. Lehman RA, Kuklo TR, Freedman BA, Cowart JR, Mense MG, Riew KD. The effect of alendronate sodium on spinal fusion: a rabbit model. Spine J. 2004. 4:36–43.

19. Glorieux FH, Bishop NJ, Plotkin H, Chabot G, Lanoue G, Travers R. Cyclic administration of pamidronate in children with severe osteogenesis imperfecta. N Engl J Med. 1998. 339:947–952.

20. Munns CF, Rauch F, Zeitlin L, Fassier F, Glorieux FH. Delayed osteotomy but not fracture healing in pediatric osteogenesis imperfecta patients receiving pamidronate. J Bone Miner Res. 2004. 19:1779–1786.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download